OMV Vaccines and the Role of TLR Agonists in Immune Response
- PMID: 32575921
- PMCID: PMC7352230
- DOI: 10.3390/ijms21124416
OMV Vaccines and the Role of TLR Agonists in Immune Response
Abstract
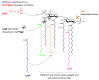
Outer Membrane Vesicles (OMVs) are bacterial nanoparticles that are spontaneously released during growth both in vitro and in vivo by Gram-negative bacteria. They are spherical, bilayered membrane nanostructures that contain many components found within the external surface of the parent bacterium. Naturally, OMVs serve the bacteria as a mechanism to deliver DNA, RNA, proteins, and toxins, as well as to promote biofilm formation and remodel the outer membrane during growth. On the other hand, as OMVs possess the optimal size to be uptaken by immune cells, and present a range of surface-exposed antigens in native conformation and Toll-like receptor (TLR) activating components, they represent an attractive and powerful vaccine platform able to induce both humoral and cell-mediated immune responses. This work reviews the TLR-agonists expressed on OMVs and their capability to trigger individual TLRs expressed on different cell types of the immune system, and then focuses on their impact on the immune responses elicited by OMVs compared to traditional vaccines.
Keywords: GMMA; PAMP; TLR; outer membrane vesicle (OMV); vaccine.
Conflict of interest statement
The authors have the following conflict of interest to declare: all authors are employees of GSK Vaccines Institute for Global Health, part of the GSK group of companies. This does not alter the Authors’ adherence to all journal policies on data and material sharing.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

