Novel Thienopyrimidine Inhibitors of Leishmania N-Myristoyltransferase with On-Target Activity in Intracellular Amastigotes
- PMID: 32575985
- PMCID: PMC7383931
- DOI: 10.1021/acs.jmedchem.0c00570
Novel Thienopyrimidine Inhibitors of Leishmania N-Myristoyltransferase with On-Target Activity in Intracellular Amastigotes
Abstract
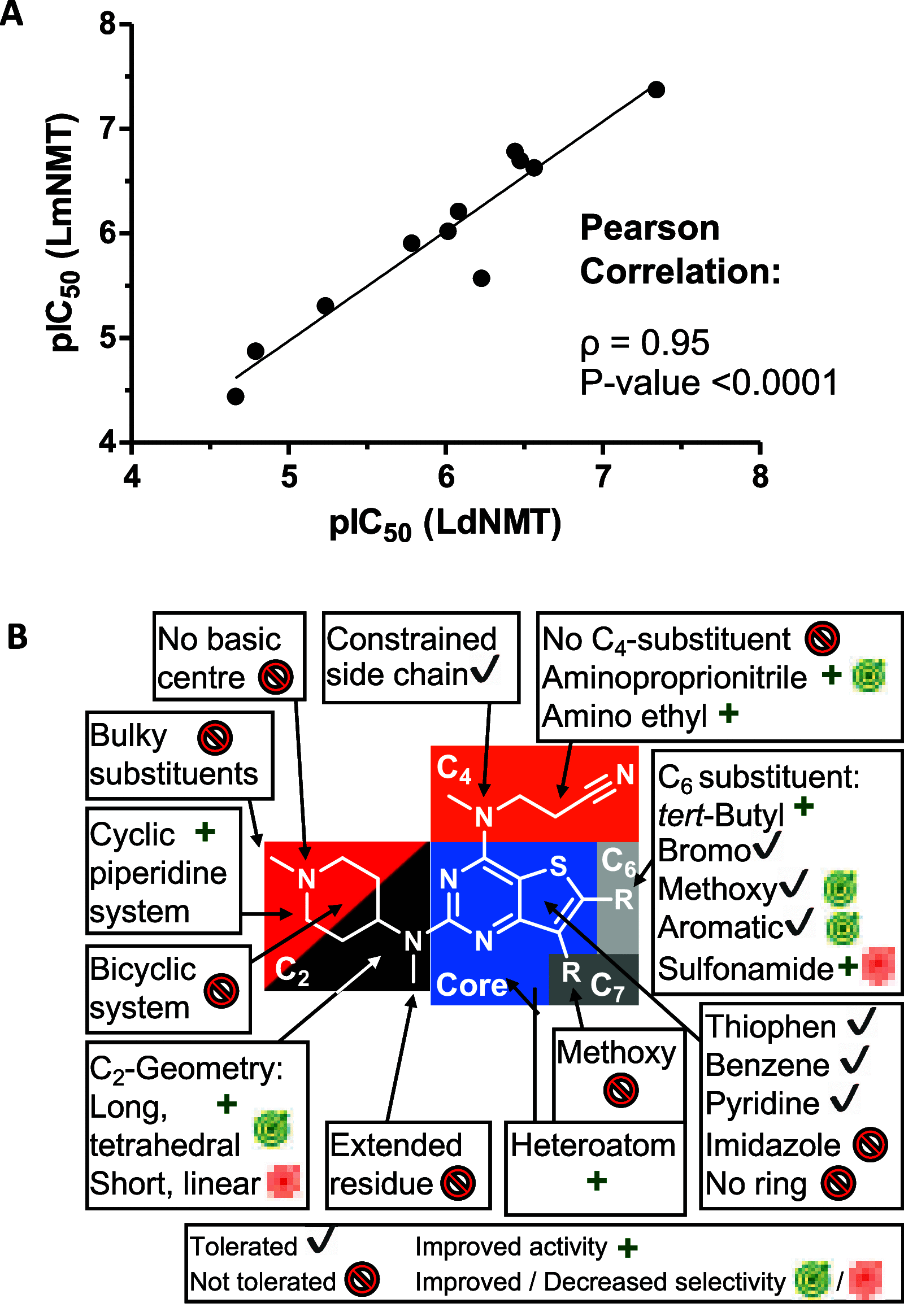
The leishmaniases, caused by Leishmania species of protozoan parasites, are neglected tropical diseases with millions of cases worldwide. Current therapeutic approaches are limited by toxicity, resistance, and cost. N-Myristoyltransferase (NMT), an enzyme ubiquitous and essential in all eukaryotes, has been validated via genetic and pharmacological methods as a promising anti-leishmanial target. Here we describe a comprehensive structure-activity relationship (SAR) study of a thienopyrimidine series previously identified in a high-throughput screen against Leishmania NMT, across 68 compounds in enzyme- and cell-based assay formats. Using a chemical tagging target engagement biomarker assay, we identify the first inhibitor in this series with on-target NMT activity in leishmania parasites. Furthermore, crystal structure analyses of 12 derivatives in complex with Leishmania major NMT revealed key factors important for future structure-guided optimization delivering IMP-105 (43), a compound with modest activity against Leishmania donovani intracellular amastigotes and excellent selectivity (>660-fold) for Leishmania NMT over human NMTs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


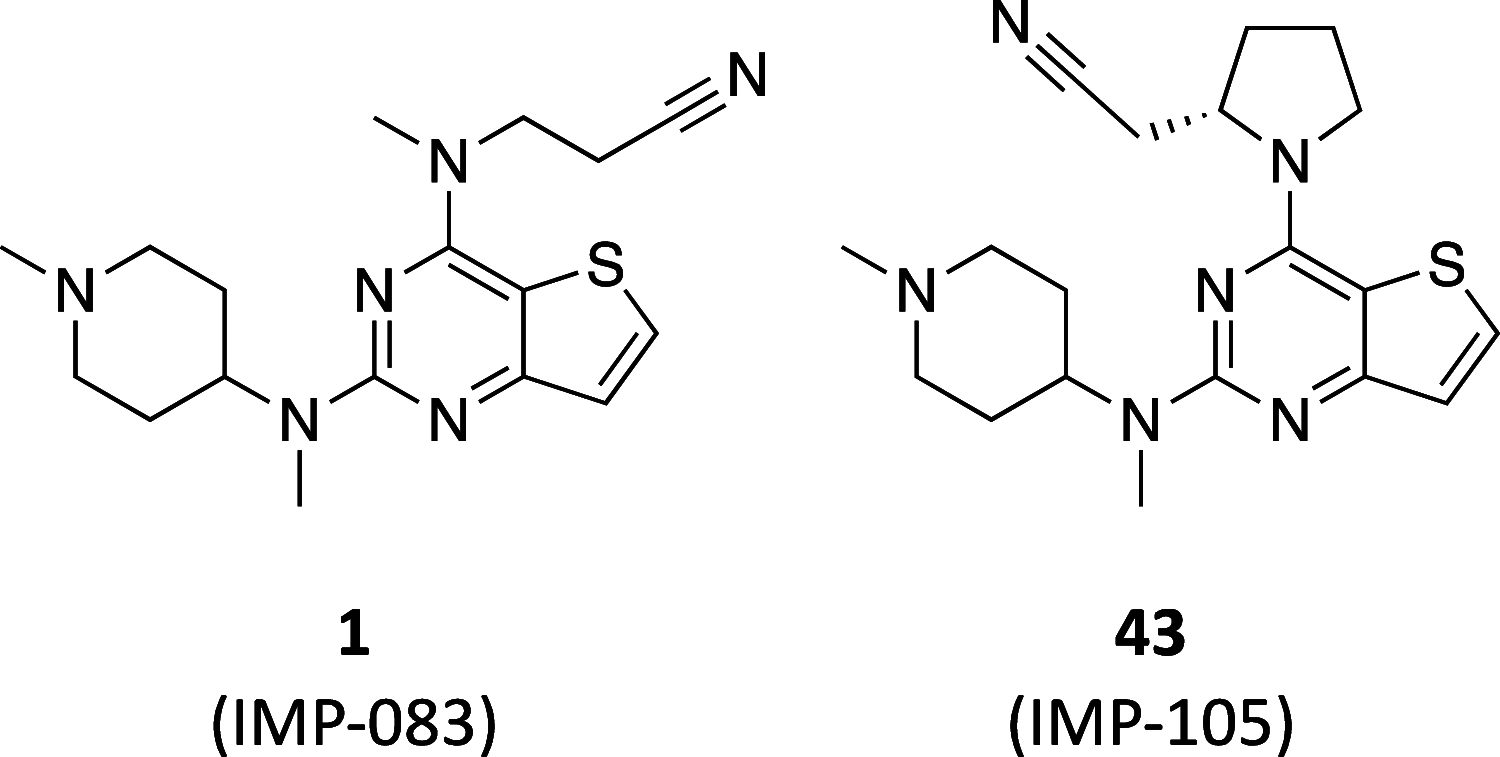
References
-
- WHO, Leishmaniasis. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed 14 June, 2020).
-
- Fang W.; Robinson D. A.; Raimi O. G.; Blair D. E.; Harrison J. R.; Lockhart D. E. A.; Torrie L. S.; Ruda G. F.; Wyatt P. G.; Gilbert I. H.; Van Aalten D. M. F. N -Myristoyltransferase Is a Cell Wall Target in Aspergillus Fumigatus. ACS Chem. Biol. 2015, 10, 1425–1434. 10.1021/cb5008647. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

