Lung and general health effects of Toll-like receptor-4 (TLR4)-interacting SPA4 peptide
- PMID: 32576172
- PMCID: PMC7310322
- DOI: 10.1186/s12890-020-01187-7
Lung and general health effects of Toll-like receptor-4 (TLR4)-interacting SPA4 peptide
Abstract
Background: A surfactant protein-A-derived peptide, which we call SPA4 peptide (amino acids: GDFRYSDGTPVNYTNWYRGE), alleviates lung infection and inflammation. This study investigated the effects of intratracheally administered SPA4 peptide on systemic, lung, and health parameters in an outbred mouse strain, and in an intratracheal lipopolysaccharide (LPS) challenge model.
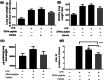
Methods: The outbred CD-1 mice were intratracheally administered with incremental doses of SPA4 peptide (0.625-10 μg/g body weight) once every 24 h, for 3 days. Mice left untreated and those treated with vehicle were included as controls. Mice were euthanized after 24 h of last administration of SPA4 peptide. In order to assess the biological activity of SPA4 peptide, C57BL6 mice were intratracheally challenged with 5 μg LPS/g body weight and treated with 50 μg SPA4 peptide via intratracheal route 1 h post LPS-challenge. Mice were euthanized after 4 h of LPS challenge. Signs of sickness and body weights were regularly monitored. At the time of necropsy, blood and major organs were harvested. Blood gas and electrolytes, serum biochemical profiles and SPA4 peptide-specific immunoglobulin G (IgG) antibody levels, and common lung injury markers (levels of total protein, albumin, and lactate, lactate dehydrogenase activity, and lung wet/dry weight ratios) were determined. Lung, liver, spleen, kidney, heart, and intestine were examined histologically. Differences in measured parameters were analyzed among study groups by analysis of variance test.
Results: The results demonstrated no signs of sickness or changes in body weight over 3 days of treatment with various doses of SPA4 peptide. It did not induce any major toxicity or IgG antibody response to SPA4 peptide. The SPA4 peptide treatment also did not affect blood gas, electrolytes, or serum biochemistry. There was no evidence of injury to the tissues and organs. However, the SPA4 peptide suppressed the LPS-induced lung inflammation.
Conclusions: These findings provide an initial toxicity profile of SPA4 peptide. Intratracheal administration of escalating doses of SPA4 peptide does not induce any significant toxicity at tissue and organ levels. However, treatment with a dose of 50 μg SPA4 peptide, comparable to 2.5 μg/g body weight, alleviates LPS-induced lung inflammation.
Keywords: Anti-inflammatory activity; Health effects; Pulmonary toxicology; Surfactant protein-A-derived peptide.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
Mechanism of Anti-Inflammatory Activity of TLR4-Interacting SPA4 Peptide.Immunohorizons. 2021 Aug 24;5(8):659-674. doi: 10.4049/immunohorizons.2100067. Immunohorizons. 2021. PMID: 34429343 Free PMC article.
-
TLR4-interacting SPA4 peptide improves host defense and alleviates tissue injury in a mouse model of Pseudomonas aeruginosa lung infection.PLoS One. 2019 Jan 28;14(1):e0210979. doi: 10.1371/journal.pone.0210979. eCollection 2019. PLoS One. 2019. PMID: 30689633 Free PMC article.
-
A TLR4-interacting SPA4 peptide inhibits LPS-induced lung inflammation.Innate Immun. 2013 Dec;19(6):596-610. doi: 10.1177/1753425912474851. Epub 2013 Mar 8. Innate Immun. 2013. PMID: 23475791
-
Effects of SPA4 peptide on lipopolysaccharide-disrupted lung epithelial barrier, injury, and function in a human cell system and mouse model of lung injury.Physiol Rep. 2022 Jul;10(13):e15353. doi: 10.14814/phy2.15353. Physiol Rep. 2022. PMID: 35838161 Free PMC article.
-
Toll-like receptor 4-interacting SPA4 peptide suppresses the NLRP3 inflammasome in response to LPS and ATP stimuli.J Leukoc Biol. 2015 Dec;98(6):1037-48. doi: 10.1189/jlb.3A1114-570R. Epub 2015 Aug 7. J Leukoc Biol. 2015. PMID: 26254306 Free PMC article.
Cited by
-
The Role of Toll-like Receptors (TLRs) Mediated Inflammation in Pancreatic Cancer Pathophysiology.Int J Mol Sci. 2021 Nov 25;22(23):12743. doi: 10.3390/ijms222312743. Int J Mol Sci. 2021. PMID: 34884547 Free PMC article. Review.
-
Revisiting the role of pulmonary surfactant in chronic inflammatory lung diseases and environmental exposure.Eur Respir Rev. 2021 Dec 15;30(162):210077. doi: 10.1183/16000617.0077-2021. Print 2021 Dec 31. Eur Respir Rev. 2021. PMID: 34911693 Free PMC article. Review.
-
Involvement of endoplasmic reticulum and histone proteins in immunomodulation by TLR4-interacting SPA4 peptide against Escherichia coli.Infect Immun. 2023 Dec 12;91(12):e0031123. doi: 10.1128/iai.00311-23. Epub 2023 Nov 1. Infect Immun. 2023. PMID: 37909750 Free PMC article.
-
Mechanism of Anti-Inflammatory Activity of TLR4-Interacting SPA4 Peptide.Immunohorizons. 2021 Aug 24;5(8):659-674. doi: 10.4049/immunohorizons.2100067. Immunohorizons. 2021. PMID: 34429343 Free PMC article.
-
Stability and structure-activity relationship of the SPA4 peptide under ambient and stressed conditions of lung injury.RSC Adv. 2023 Jun 21;13(27):18864-18877. doi: 10.1039/d3ra02918b. eCollection 2023 Jun 15. RSC Adv. 2023. PMID: 37350860 Free PMC article.
References
-
- Fan J, Li Y, Vodovotz Y, Billiar TR, Wilson MA. Hemorrhagic shock-activated neutrophils augment TLR4 signaling-induced TLR2 upregulation in alveolar macrophages: role in hemorrhage-primed lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L738–L746. doi: 10.1152/ajplung.00280.2005. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

