Pre-implementation adaptation of primary care cancer prevention clinical decision support in a predominantly rural healthcare system
- PMID: 32576202
- PMCID: PMC7310565
- DOI: 10.1186/s12911-020-01136-8
Pre-implementation adaptation of primary care cancer prevention clinical decision support in a predominantly rural healthcare system
Abstract
Background: Cancer is a leading cause of death in the United States. Primary care providers (PCPs) juggle patient cancer prevention and screening along with managing acute and chronic health problems. However, clinical decision support (CDS) may assist PCPs in addressing patients' cancer prevention and screening needs during short clinic visits. In this paper, we describe pre-implementation study design and cancer screening and prevention CDS changes made to maximize utilization and better fit a healthcare system's goals and culture. We employed the Consolidated Framework for Implementation Research (CFIR), useful for evaluating the implementation of CDS interventions in primary care settings, in understanding barriers and facilitators that led to those changes.
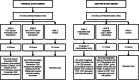
Methods: In a three-arm, pragmatic, 36 clinic cluster-randomized control trial, we integrated cancer screening and prevention CDS and shared decision-making tools (SDMT) into an existing electronic medical record-linked cardiovascular risk management CDS system. The integrated CDS is currently being tested within a predominately rural upper Midwestern healthcare system. Prior to CDS implementation, we catalogued pre-implementation changes made from 2016 to 2018 based on: pre-implementation site engagement; key informant interviews with healthcare system rooming staff, providers, and leadership; and pilot testing. We identified influential barriers, facilitators, and changes made in response through qualitative content analysis of meeting minutes and supportive documents. We then coded pre-implementation changes made and associated barriers and facilitators using the CFIR.
Results: Based on our findings from system-wide pre-implementation engagement, pilot testing, and key informant interviews, we made changes to accommodate the needs of the healthcare system based on barriers and facilitators that fell within the Intervention Characteristics, Inner Setting, and Outer Setting CFIR domains. Changes included replacing the expansion of medical assistant roles in one intervention arm with targeted SDMT, as well as altering cancer prevention CDS and study design elements.
Conclusions: Pre-implementation changes to CDS may help meet healthcare systems' evolving needs and optimize the intervention by being responsive to real-world implementation barriers and facilitators. Frameworks like the CFIR are useful tools for identifying areas where pre-implementation barriers and facilitators may result in design changes, both to research studies and CDS systems.
Trial registration: NCT02986230.
Keywords: Cancer prevention and screening; Clinical decision support; Consolidated Framework for Implementation Research; Pre-implementation adaptation; Primary care; Shared decision-making tools.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Office of Disease Prevention and Health Promotion. Cancer. Office of Disease Prevention and Health Promotion website. 2019. https://www.healthypeople.gov/2020/topics-objectives/topic/cancer. Accessed 29 May 2020.
-
- Barrett B, McKenna P. Communicating benefits and risks of screening for prostate, colon, and breast cancer. Fam Med 2011;43:248–53. - PubMed
-
- Petrova D, Garcia-Retamero R, Cokely ET. Understanding the harms and benefits of cancer screening: a model of factors that shape informed decision making. Med Decis Mak 2015;35:847–858. https://doi.org/10.1177/0272989X15587676. - PubMed
-
- Hoffman RM, Lewis CL, Pignone MP, Couper MP, Barry MJ, Elmore JG, et al. Decision-making processes for breast, colorectal, and prostate cancer screening: the DECISIONS survey. Med Decis Mak 2010;30:53S–64S. https://doi.org/10.1177/0272989X10378701. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

