Effect of age on pharmacokinetics, efficacy, and safety of galcanezumab treatment in adult patients with migraine: results from six phase 2 and phase 3 randomized clinical trials
- PMID: 32576229
- PMCID: PMC7310276
- DOI: 10.1186/s10194-020-01148-9
Effect of age on pharmacokinetics, efficacy, and safety of galcanezumab treatment in adult patients with migraine: results from six phase 2 and phase 3 randomized clinical trials
Abstract
Background: Migraine clinical profile may change with age, making it necessary to verify that migraine treatments are equally safe and effective in older patients. These analyses evaluated the effects of patient age on the pharmacokinetics (PK), efficacy, and safety of galcanezumab for prevention of migraine.
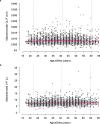
Methods: Analyses included efficacy data from three double-blind phase 3 clinical trials: two 6-month studies in episodic migraine (EVOLVE-1, EVOLVE-2: N = 1773) and one 3-month study in chronic migraine (REGAIN:N = 1113). Patients were randomized 2:1:1 to placebo, galcanezumab 120 mg, or galcanezumab 240 mg. Safety and PK data included additional phase 2 and phase 3 trials for a larger sample size of patients > 60 years (range = 18-65 for all studies). Subgroup analyses assessed efficacy measures, adverse event (AE) occurrence, and cardiovascular measurement changes by patient age group. Galcanezumab PK were evaluated using a population analysis approach, where age was examined as a potential covariate on apparent clearance (CL/F) and apparent volume of distribution (V/F) of galcanezumab.
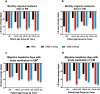
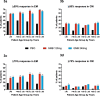
Results: Numbers of baseline monthly migraine headache days were similar across age groups. There were no statistically significant treatment-by-age group interactions for any efficacy measures, except in episodic migraine studies where older patients appeared to have a larger reduction than younger patients in the number of monthly migraine headache days with acute medication use. Age (18-65) had a minimal effect on CL/F, and no effect on V/F. Galcanezumab-treated patients ≥60 years experienced no clinically meaningful increases in blood pressure and no increased frequency in treatment-emergent AEs, discontinuations due to AEs, serious adverse events (SAEs) overall, or cardiovascular SAEs, compared to age-matched placebo-treated patients.
Conclusions: Age (up to 65 years) does not affect efficacy in migraine prevention and has no clinically meaningful influence on galcanezumab PK to warrant dose adjustment. Furthermore, older galcanezumab-treated patients experienced no increases in frequency of AEs or increases in blood pressure compared with age-matched placebo-treated patients.
Trial registrations: EVOLVE-1 (NCT02614183, registered 23 November 2015), EVOLVE-2 (NCT02614196, 23 November 2015), REGAIN (NCT02614261, 23 November 2015), ART-01 (NCT01625988, 20 June 2012, ), I5Q-MC-CGAB (NCT02163993, 12 June 2014, ), I5Q-MC-CGAJ (NCT02614287, 23 November 2015, ), all retrospectively registered.
Keywords: Aging population; CGRP antagonist; Elderly; Galcanezumab; Migraine; Migraine in older adults; Migraine prevention; Migraine prophylaxis; Monoclonal antibody.
Conflict of interest statement
VLS is an employee of Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA and is a minor stockholder of Eli Lilly and Company.
IT has received grants from Allergan, Amgen, Biohaven, ElectroCore, Eli Lilly and Company, Lundbeck (Alder), Nerivio, and Teva. He has received payments for speaking and/or consulting from Allergan, Amgen, Biohaven, Eli Lilly and Company, Lundbeck, Headache Cooperative of New England, Nerivio, Novartis, Revance, Teva, and The Headache Institute. IT received non-financial support from Eli Lilly and Company.
PK is an employee of Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA and is a minor stockholder of Eli Lilly and Company.
WK is an employee of Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA and is a minor stockholder of Eli Lilly and Company.
KD is an employee of Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA and is a minor stockholder of Eli Lilly and Company.
MP is an employee of Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA and is a minor stockholder of Eli Lilly and Company.
TQ is an employee of Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA and is a minor stockholder of Eli Lilly and Company.
AC is an employee of Eli Lilly and Company and/or one of its subsidiaries, Indianapolis, IN, USA and is a minor stockholder of Eli Lilly and Company.
Figures
References
-
- Eli Lilly and Company . Emgality Highlights of Prescribing Information. 2018.
-
- Eli Lilly and Company . Emgality Summary of Product Characteristics. 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials