Discharge and Role of GABA Pontomesencephalic Neurons in Cortical Activity and Sleep-Wake States Examined by Optogenetics and Juxtacellular Recordings in Mice
- PMID: 32576622
- PMCID: PMC7392501
- DOI: 10.1523/JNEUROSCI.2875-19.2020
Discharge and Role of GABA Pontomesencephalic Neurons in Cortical Activity and Sleep-Wake States Examined by Optogenetics and Juxtacellular Recordings in Mice
Abstract
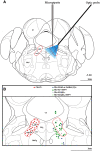
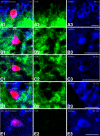
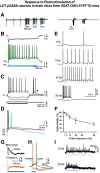
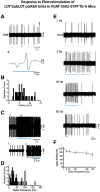
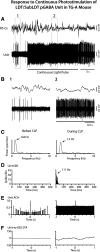
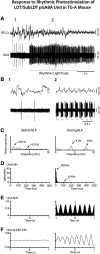
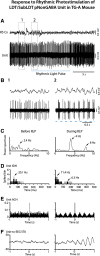
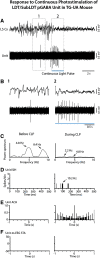
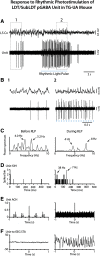
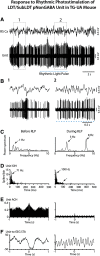
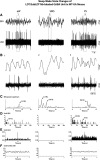
The cholinergic neurons in the pontomesencephalic tegmentum have been shown to discharge in association with and promote cortical activation during active or attentive waking and paradoxical or rapid eye movement sleep. However, GABA neurons lie intermingled with the cholinergic neurons and may contribute to or oppose this activity and role. Here we investigated in vitro and in vivo the properties, activities, and role of GABA neurons within the laterodorsal tegmental and sublaterodorsal tegmental nuclei (LDT/SubLDT) using male and female transgenic mice expressing channelrhodopsin-(ChR2)-EYFP in vesicular GABA transporter (VGAT)-expressing neurons. Presumed GABA (pGABA) neurons were identified by response to photostimulation and verified by immunohistochemical staining following juxtacellular labeling in vivo pGABA neurons were found to be fast-firing neurons with the capacity to burst when depolarized from a hyperpolarized membrane potential. When stimulated in vivo in urethane-anesthetized or unanesthetized mice, the pGABA neurons fired repetitively at relatively fast rates (∼40 Hz) during a continuous light pulse or phasically in bursts (>100 Hz) when driven by rhythmic light pulses at theta (4 or 8 Hz) frequencies. pNon-GABA, which likely included cholinergic, neurons were inhibited during each light pulse to discharge rhythmically in antiphase to the pGABA neurons. The reciprocal rhythmic bursting by the pGABA and pNon-GABA neurons drove rhythmic theta activity in the EEG. Such phasic bursting by GABA neurons also occurred in WT mice in association with theta activity during attentive waking and paradoxical sleep.SIGNIFICANCE STATEMENT Neurons in the pontomesencephalic tegmentum, particularly cholinergic neurons, play an important role in cortical activation, which occurs during active or attentive waking and paradoxical or rapid eye movement sleep. Yet the cholinergic neurons lie intermingled with GABA neurons, which could play a similar or opposing role. Optogenetic stimulation and recording of these GABA neurons in mice revealed that they can discharge in rhythmic bursts at theta frequencies and drive theta activity in limbic cortex. Such phasic burst firing also occurs during natural attentive waking and paradoxical sleep in association with theta activity and could serve to enhance sensory-motor processing and memory consolidation during these states.
Keywords: EEG; REM sleep; paradoxical sleep; slow wave sleep; theta; waking.
Copyright © 2020 the authors.
Figures

Similar articles
-
Discharge and Role of Acetylcholine Pontomesencephalic Neurons in Cortical Activity and Sleep-Wake States Examined by Optogenetics and Juxtacellular Recording in Mice.eNeuro. 2018 Sep 13;5(4):ENEURO.0270-18.2018. doi: 10.1523/ENEURO.0270-18.2018. eCollection 2018 Jul-Aug. eNeuro. 2018. PMID: 30225352 Free PMC article.
-
Discharge properties of presumed cholinergic and noncholinergic laterodorsal tegmental neurons related to cortical activation in non-anesthetized mice.Neuroscience. 2012 Nov 8;224:172-90. doi: 10.1016/j.neuroscience.2012.08.032. Epub 2012 Aug 21. Neuroscience. 2012. PMID: 22917614
-
Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat.J Neurosci. 2014 Mar 26;34(13):4708-27. doi: 10.1523/JNEUROSCI.2617-13.2014. J Neurosci. 2014. PMID: 24672016 Free PMC article.
-
Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex.Prog Brain Res. 2004;145:157-69. doi: 10.1016/S0079-6123(03)45011-5. Prog Brain Res. 2004. PMID: 14650914 Review.
-
Paradoxical sleep and its chemical/structural substrates in the brain.Neuroscience. 1991;40(3):637-56. doi: 10.1016/0306-4522(91)90002-6. Neuroscience. 1991. PMID: 2062436 Review.
Cited by
-
The regulation of the pedunculopontine tegmental nucleus in sleep-wake states.Sleep Biol Rhythms. 2023 Sep 26;22(1):5-11. doi: 10.1007/s41105-023-00489-7. eCollection 2024 Jan. Sleep Biol Rhythms. 2023. PMID: 38469582 Free PMC article. Review.
-
Diverse Firing Profiles of Crhbp-positive Neurons in the Dorsal Pons Suggestive of Their Pleiotropic Roles in REM Sleep Regulation in Mice.J Neurosci. 2025 Jul 9;45(28):e2365242025. doi: 10.1523/JNEUROSCI.2365-24.2025. J Neurosci. 2025. PMID: 40550695
-
Understanding Sleep Regulation in Normal and Pathological Conditions, and Why It Matters.J Huntingtons Dis. 2023;12(2):105-119. doi: 10.3233/JHD-230564. J Huntingtons Dis. 2023. PMID: 37302038 Free PMC article. Review.
-
Pontine control of rapid eye movement sleep and fear memory.CNS Neurosci Ther. 2023 Jun;29(6):1602-1614. doi: 10.1111/cns.14123. Epub 2023 Feb 16. CNS Neurosci Ther. 2023. PMID: 36794544 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
