The orphan receptor GPR139 signals via Gq/11 to oppose opioid effects
- PMID: 32576659
- PMCID: PMC7397111
- DOI: 10.1074/jbc.AC120.014770
The orphan receptor GPR139 signals via Gq/11 to oppose opioid effects
Abstract
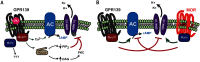
The interplay between G protein-coupled receptors (GPCRs) is critical for controlling neuronal activity that shapes neuromodulatory outcomes. Recent evidence indicates that the orphan receptor GPR139 influences opioid modulation of key brain circuits by opposing the actions of the µ-opioid receptor (MOR). However, the function of GPR139 and its signaling mechanisms are poorly understood. In this study, we report that GPR139 activates multiple heterotrimeric G proteins, including members of the Gq/11 and Gi/o families. Using a panel of reporter assays in reconstituted HEK293T/17 cells, we found that GPR139 functions via the Gq/11 pathway and thereby distinctly regulates cellular effector systems, including stimulation of cAMP production and inhibition of G protein inward rectifying potassium (GIRK) channels. Electrophysiological recordings from medial habenular neurons revealed that GPR139 signaling via Gq/11 is necessary and sufficient for counteracting MOR-mediated inhibition of neuronal firing. These results uncover a mechanistic interplay between GPCRs involved in controlling opioidergic neuromodulation in the brain.
Keywords: G protein-coupled receptor (GPCR); G protein-coupled receptor 139 (GPR139); GIRK channel; GPCR signaling; adenylate cyclase (adenylyl cyclase); brain; cell signaling; cellular regulation; heterotrimeric G protein; medial habenula; neuron; opiate opioid; opioids; orphan receptor.
© 2020 Stoveken et al.
Conflict of interest statement
Conflict of interest—B. G. and K. A. M. have filed a patent on the utility of GPR139 as a drug target.
Figures




References
-
- Wang D., Stoveken H. M., Zucca S., Dao M., Orlandi C., Song C., Masuho I., Johnston C., Opperman K. J., Giles A. C., Gill M. S., Lundquist E. A., Grill B., and Martemyanov K. A. (2019) Genetic behavioral screen identifies an orphan anti-opioid system. Science 365, 1267–1273 10.1126/science.aau2078 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

