Roles of the EnvZ/OmpR Two-Component System and Porins in Iron Acquisition in Escherichia coli
- PMID: 32576675
- PMCID: PMC7315122
- DOI: 10.1128/mBio.01192-20
Roles of the EnvZ/OmpR Two-Component System and Porins in Iron Acquisition in Escherichia coli
Abstract
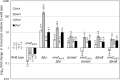
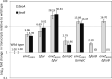
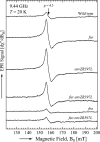
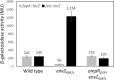
Escherichia coli secretes high-affinity Fe3+ chelators to solubilize and transport chelated Fe3+ via specific outer membrane receptors. In microaerobic and anaerobic growth environments, where the reduced Fe2+ form is predominant, ferrous transport systems fulfill the bacterial need for iron. Expression of genes coding for iron metabolism is controlled by Fur, which when bound to Fe2+ acts as a repressor. Work carried out here shows that the constitutively activated EnvZ/OmpR two-component system, which normally controls expression of the ompC and ompF porin genes, dramatically increases the intracellular pool of accessible iron, as determined by whole-cell electron paramagnetic resonance spectroscopy, by inducing the OmpC/FeoB-mediated ferrous transport pathway. Elevated levels of intracellular iron in turn activated Fur, which inhibited the ferric transport pathway but not the ferrous transport pathway. The data show that the positive effect of constitutively activated EnvZ/OmpR on feoB expression is sufficient to overcome the negative effect of activated Fur on feoB In a tonB mutant, which lacks functional ferric transport systems, deletion of ompR severely impairs growth on rich medium not supplemented with iron, while the simultaneous deletion of ompC and ompF is not viable. These data, together with the observation of derepression of the Fur regulon in an OmpC mutant, show that the porins play an important role in iron homeostasis. The work presented here also resolves a long-standing paradoxical observation of the effect of certain mutant envZ alleles on iron regulon.IMPORTANCE The work presented here solved a long-standing paradox of the negative effects of certain missense alleles of envZ, which codes for kinase of the EnvZ/OmpR two-component system, on the expression of ferric uptake genes. The data revealed that the constitutive envZ alleles activate the Feo- and OmpC-mediated ferrous uptake pathway to flood the cytoplasm with accessible ferrous iron. This activates the ferric uptake regulator, Fur, which inhibits ferric uptake system but cannot inhibit the feo operon due to the positive effect of activated EnvZ/OmpR. The data also revealed the importance of porins in iron homeostasis.
Keywords: EnvZ/OmpR; ferric transport; ferrous transport; iron homeostasis; porins; two-component signal transduction systems.
Copyright © 2020 Gerken et al.
Figures


Similar articles
-
Identification of ethanol tolerant outer membrane proteome reveals OmpC-dependent mechanism in a manner of EnvZ/OmpR regulation in Escherichia coli.J Proteomics. 2018 May 15;179:92-99. doi: 10.1016/j.jprot.2018.03.005. Epub 2018 Mar 6. J Proteomics. 2018. PMID: 29518576
-
Effects of pleiotropic ompR and envZ alleles of Escherichia coli on envelope stress and antibiotic sensitivity.J Bacteriol. 2024 Jun 20;206(6):e0017224. doi: 10.1128/jb.00172-24. Epub 2024 May 29. J Bacteriol. 2024. PMID: 38809006 Free PMC article.
-
MzrA: a novel modulator of the EnvZ/OmpR two-component regulon.Mol Microbiol. 2009 Jun;72(6):1408-22. doi: 10.1111/j.1365-2958.2009.06728.x. Epub 2009 May 8. Mol Microbiol. 2009. PMID: 19432797 Free PMC article.
-
EnvZ/OmpR Two-Component Signaling: An Archetype System That Can Function Noncanonically.EcoSal Plus. 2020 Jan;9(1):10.1128/ecosalplus.ESP-0001-2019. doi: 10.1128/ecosalplus.ESP-0001-2019. EcoSal Plus. 2020. PMID: 32003321 Free PMC article. Review.
-
Signal transduction and gene regulation through the phosphorylation of two regulatory components: the molecular basis for the osmotic regulation of the porin genes.Mol Microbiol. 1990 Jul;4(7):1077-82. doi: 10.1111/j.1365-2958.1990.tb00681.x. Mol Microbiol. 1990. PMID: 1700256 Review.
Cited by
-
The sbiTRS Operon Contributes to Stenobactin-Mediated Iron Utilization in Stenotrophomonas maltophilia.Microbiol Spectr. 2022 Dec 21;10(6):e0267322. doi: 10.1128/spectrum.02673-22. Epub 2022 Dec 1. Microbiol Spectr. 2022. PMID: 36453931 Free PMC article.
-
Molecular Characteristics and Quantitative Proteomic Analysis of Klebsiella pneumoniae Strains with Carbapenem and Colistin Resistance.Antibiotics (Basel). 2022 Sep 30;11(10):1341. doi: 10.3390/antibiotics11101341. Antibiotics (Basel). 2022. PMID: 36289999 Free PMC article.
-
Reactive oxygen species accelerate de novo acquisition of antibiotic resistance in E. coli.iScience. 2023 Oct 31;26(12):108373. doi: 10.1016/j.isci.2023.108373. eCollection 2023 Dec 15. iScience. 2023. PMID: 38025768 Free PMC article.
-
Two-component systems regulate bacterial virulence in response to the host gastrointestinal environment and metabolic cues.Virulence. 2022 Dec;13(1):1666-1680. doi: 10.1080/21505594.2022.2127196. Virulence. 2022. PMID: 36128741 Free PMC article. Review.
-
A novel esterase regulates Klebsiella pneumoniae hypermucoviscosity and virulence.PLoS Pathog. 2024 Oct 31;20(10):e1012675. doi: 10.1371/journal.ppat.1012675. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39480904 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
