Upregulation of CD47 Is a Host Checkpoint Response to Pathogen Recognition
- PMID: 32576678
- PMCID: PMC7315125
- DOI: 10.1128/mBio.01293-20
Upregulation of CD47 Is a Host Checkpoint Response to Pathogen Recognition
Abstract
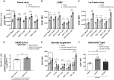
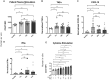
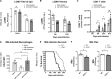
It is well understood that the adaptive immune response to infectious agents includes a modulating suppressive component as well as an activating component. We now show that the very early innate response also has an immunosuppressive component. Infected cells upregulate the CD47 "don't eat me" signal, which slows the phagocytic uptake of dying and viable cells as well as downstream antigen-presenting cell (APC) functions. A CD47 mimic that acts as an essential virulence factor is encoded by all poxviruses, but CD47 expression on infected cells was found to be upregulated even by pathogens, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that encode no mimic. CD47 upregulation was revealed to be a host response induced by the stimulation of both endosomal and cytosolic pathogen recognition receptors (PRRs). Furthermore, proinflammatory cytokines, including those found in the plasma of hepatitis C patients, upregulated CD47 on uninfected dendritic cells, thereby linking innate modulation with downstream adaptive immune responses. Indeed, results from antibody-mediated CD47 blockade experiments as well as CD47 knockout mice revealed an immunosuppressive role for CD47 during infections with lymphocytic choriomeningitis virus and Mycobacterium tuberculosis Since CD47 blockade operates at the level of pattern recognition receptors rather than at a pathogen or antigen-specific level, these findings identify CD47 as a novel potential immunotherapeutic target for the enhancement of immune responses to a broad range of infectious agents.IMPORTANCE Immune responses to infectious agents are initiated when a pathogen or its components bind to pattern recognition receptors (PRRs). PRR binding sets off a cascade of events that activates immune responses. We now show that, in addition to activating immune responses, PRR signaling also initiates an immunosuppressive response, probably to limit inflammation. The importance of the current findings is that blockade of immunomodulatory signaling, which is mediated by the upregulation of the CD47 molecule, can lead to enhanced immune responses to any pathogen that triggers PRR signaling. Since most or all pathogens trigger PRRs, CD47 blockade could be used to speed up and strengthen both innate and adaptive immune responses when medically indicated. Such immunotherapy could be done without a requirement for knowing the HLA type of the individual, the specific antigens of the pathogen, or, in the case of bacterial infections, the antimicrobial resistance profile.
Keywords: CD47; host response; immune checkpoint; innate immunity; pathogen recognition receptors.
Figures


References
-
- Wei SC, Anang N-AAS, Sharma R, Andrews MC, Reuben A, Levine JH, Cogdill AP, Mancuso JJ, Wargo JA, Pe’er D, Allison JP. 2019. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U S A 116:22699–22709. doi:10.1073/pnas.1821218116. - DOI - PMC - PubMed
-
- Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, Tran T, Lynn J, Chen JY, Volkmer J-P, Agoram B, Huang J, Majeti R, Weissman IL, Takimoto CH, Chao MP, Smith SM. 2018. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med 379:1711–1721. doi:10.1056/NEJMoa1807315. - DOI - PMC - PubMed
-
- Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, Rasco D, Colevas AD, O’Rourke T, Narayanan S, Papadopoulos K, Fisher GA, Villalobos V, Prohaska SS, Howard M, Beeram M, Chao MP, Agoram B, Chen JY, Huang J, Axt M, Liu J, Volkmer J-P, Majeti R, Weissman IL, Takimoto CH, Supan D, Wakelee HA, Aoki R, Pegram MD, Padda SK. 2019. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol 37:946–953. doi:10.1200/JCO.18.02018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
