TLR2 on blood monocytes senses dengue virus infection and its expression correlates with disease pathogenesis
- PMID: 32576819
- PMCID: PMC7311456
- DOI: 10.1038/s41467-020-16849-7
TLR2 on blood monocytes senses dengue virus infection and its expression correlates with disease pathogenesis
Abstract
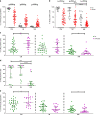
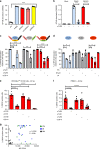
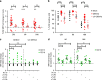
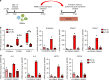
Vascular permeability and plasma leakage are immune-pathologies of severe dengue virus (DENV) infection, but the mechanisms underlying the exacerbated inflammation during DENV pathogenesis are unclear. Here, we demonstrate that TLR2, together with its co-receptors CD14 and TLR6, is an innate sensor of DENV particles inducing inflammatory cytokine expression and impairing vascular integrity in vitro. Blocking TLR2 prior to DENV infection in vitro abrogates NF-κB activation while CD14 and TLR6 block has a moderate effect. Moreover, TLR2 block prior to DENV infection of peripheral blood mononuclear cells prevents activation of human vascular endothelium, suggesting a potential role of the TLR2-responses in vascular integrity. TLR2 expression on CD14 + + classical monocytes isolated in an acute phase from DENV-infected pediatric patients correlates with severe disease development. Altogether, these data identify a role for TLR2 in DENV infection and provide insights into the complex interaction between the virus and innate receptors that may underlie disease pathogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Immunomodulation in dengue: towards deciphering dengue severity markers.Cell Commun Signal. 2024 Sep 26;22(1):451. doi: 10.1186/s12964-024-01779-4. Cell Commun Signal. 2024. PMID: 39327552 Free PMC article. Review.
-
Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leucocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever.Immunology. 2010 Jun;130(2):202-16. doi: 10.1111/j.1365-2567.2009.03224.x. Epub 2010 Jan 27. Immunology. 2010. PMID: 20113369 Free PMC article.
-
TLR2 axis on peripheral blood mononuclear cells regulates inflammatory responses to non-infectious immature dengue virus particles.PLoS Pathog. 2022 Oct 14;18(10):e1010499. doi: 10.1371/journal.ppat.1010499. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36240261 Free PMC article.
-
The herpes simplex virus 1-encoded envelope glycoprotein B activates NF-κB through the Toll-like receptor 2 and MyD88/TRAF6-dependent signaling pathway.PLoS One. 2013;8(1):e54586. doi: 10.1371/journal.pone.0054586. Epub 2013 Jan 28. PLoS One. 2013. PMID: 23382920 Free PMC article.
-
Role of Monocytes in the Pathogenesis of Dengue.Arch Immunol Ther Exp (Warsz). 2019 Feb;67(1):27-40. doi: 10.1007/s00005-018-0525-7. Epub 2018 Sep 20. Arch Immunol Ther Exp (Warsz). 2019. PMID: 30238127 Review.
Cited by
-
Immunomodulation in dengue: towards deciphering dengue severity markers.Cell Commun Signal. 2024 Sep 26;22(1):451. doi: 10.1186/s12964-024-01779-4. Cell Commun Signal. 2024. PMID: 39327552 Free PMC article. Review.
-
Increased TNF-α Initiates Cytoplasmic Vacuolization in Whole Blood Coculture with Dengue Virus.J Immunol Res. 2021 May 6;2021:6654617. doi: 10.1155/2021/6654617. eCollection 2021. J Immunol Res. 2021. PMID: 34041302 Free PMC article.
-
A novel method for characterizing cell-cell interactions at single-cell resolution reveals unique signatures in blood T cell-monocyte complexes during infection.bioRxiv [Preprint]. 2024 Sep 23:2024.09.20.612103. doi: 10.1101/2024.09.20.612103. bioRxiv. 2024. PMID: 39386643 Free PMC article. Preprint.
-
Identification of TLR2 as a Key Target in Neuroinflammation in Vascular Dementia.Front Genet. 2022 Jul 6;13:860122. doi: 10.3389/fgene.2022.860122. eCollection 2022. Front Genet. 2022. PMID: 35873459 Free PMC article.
-
Antibody fucosylation predicts disease severity in secondary dengue infection.Science. 2021 Jun 4;372(6546):1102-1105. doi: 10.1126/science.abc7303. Science. 2021. PMID: 34083490 Free PMC article.
References
-
- World Health Organization. Dengue control. http://www.who.int/denguecontrol/disease/en/ (2018).
-
- WHO (World Health Organisation). Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd edn, 103–117 (World Health Organisation, Geneva, 1997).
-
- Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B. Cardiovascular manifestations of the emerging dengue pandemic. Nat. Rev. Cardiol. 2014;11:335–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

