Mesenchymal Stromal Cell Bioreactor for Ex Vivo Reprogramming of Human Immune Cells
- PMID: 32576889
- PMCID: PMC7311545
- DOI: 10.1038/s41598-020-67039-w
Mesenchymal Stromal Cell Bioreactor for Ex Vivo Reprogramming of Human Immune Cells
Erratum in
-
Author Correction: Mesenchymal Stromal Cell Bioreactor for Ex Vivo Reprogramming of Human Immune Cells.Sci Rep. 2020 Sep 17;10(1):15451. doi: 10.1038/s41598-020-72947-y. Sci Rep. 2020. PMID: 32943743 Free PMC article.
Abstract
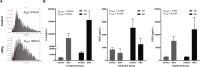
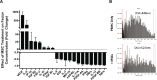
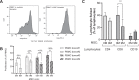
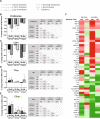
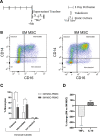
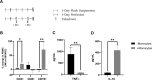
Bone marrow mesenchymal stromal cells (MSCs) have been studied for decades as potent immunomodulators. Clinically, they have shown some promise but with limited success. Here, we report the ability of a scalable hollow fiber bioreactor to effectively maintain ideal MSC function as a single population while also being able to impart an immunoregulatory effect when cultured in tandem with an inflamed lymphocyte population. MSCs were seeded on the extraluminal side of hollow fibers within a bioreactor where they indirectly interact with immune cells flowing within the lumen of the fibers. MSCs showed a stable and predictable metabolite and secreted factor profile during several days of perfusion culture. Exposure of bioreactor-seeded MSCs to inflammatory stimuli reproducibly switched MSC secreted factor profiles and altered microvesicle composition. Furthermore, circulating, activated human peripheral blood mononuclear cells (PBMCs) were suppressed by MSC bioreactor culture confirmed by a durable change in their immunophenotype and function. This platform was useful to study a model of immobilized MSCs and circulating immune cells and showed that monocytes play an important role in MSC driven immunomodulation. This coculture technology can have broad implications for use in studying MSC-immune interactions under flow conditions as well as in the generation of ex vivo derived immune cellular therapeutics.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

