GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches
- PMID: 32576980
- PMCID: PMC7309428
- DOI: 10.1038/s41577-020-0357-7
GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches
Abstract
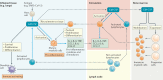
Therapeutics against coronavirus disease 2019 (COVID-19) are urgently needed. Granulocyte-macrophage colony-stimulating factor (GM-CSF), a myelopoietic growth factor and pro-inflammatory cytokine, plays a critical role in alveolar macrophage homeostasis, lung inflammation and immunological disease. Both administration and inhibition of GM-CSF are currently being therapeutically tested in COVID-19 clinical trials. This Perspective discusses the pleiotropic biology of GM-CSF and the scientific merits behind these contrasting approaches.
Conflict of interest statement
F.M.L. is a full-time employee of Roivant. Roivant is developing gimsilumab, a mAb to GM-CSF under investigation in a phase II clinical trial for the treatment of patients with COVID-19 with lung injury or ARDS. J.R.T., B.B. and J.A.H have received consulting fees from Roivant. The employer of J.A.H. and K.M.-C.L, the University of Melbourne, has licensed patented technology relating to therapeutically targeting GM-CSF to MorphoSys AG, Germany. The employer of B.B., the University of Zurich, holds a patent on the use of neutralizing GM-CSF in acute GvHD following stem cell transplantation and has a license agreement with Humanigen Inc., which is manufacturing such a GM-CSF-neutralizing mAb.
Figures
References
-
- Hamilton JA, Cook AD, Tak PP. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat. Rev. Drug. Discov. 2016;16:53–70. - PubMed
-
- Humanigen. FDA approves initiation of Humanigen’s phase III study of lenzilumab in COVID-19 patients. Humanigenhttps://www.humanigen.com/press/FDA-Approves-Initiation-of-Humanigen%E2%... (2020).
-
- I-Mab Biopharma. I-Mab announces IND clearance from FDA for TJM2 to treat cytokine release syndrome (CRS) associated with severe coronavirus disease 19 (COVID-19). I-Mab Biopharmahttp://www.i-mabbiopharma.com/en/article-517.aspx (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

