Electrospun PLGA and β-TCP (Rebossis-85) in a Lapine Posterolateral Fusion Model
- PMID: 32577102
- PMCID: PMC7047293
Electrospun PLGA and β-TCP (Rebossis-85) in a Lapine Posterolateral Fusion Model
Abstract
Background: Calcium phosphate materials have been employed clinically as bone void fillers for several decades. These materials are most often provided in the form of small, porous granules that can be packed to fill the wide variety of size and shape of bony defects encountered. ReBOSSIS-85 (RB-85) is a synthetic bioresorbable bone void filler for the repair of bone defects with handling characteristics of glass wool-like (or cotton ball-like). The objective of this study is to evaluate the in vivo performance of RB-85 (test material), compared to a commercially available bone void filler, Mastergraft Putty (predicate material), when combined with bone marrow aspirate and iliac crest autograft, in an established posterolateral spine fusion rabbit model.
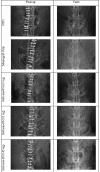
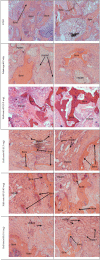
Methods: One hundred fifty skeletally mature rabbits had a single level posterolateral fusion performed. Rabbits were implanted with iliac crest bone graft (ICBG), Mastergraft Putty™ plus ICBG, or one of 4 masses of ReBOSSIS-85 (0.2, 0.3, 0.45, or 0.6 g) plus ICBG. Plain films were taken weekly until euthanasia. Following euthanasia at 4, 8, and 12 weeks, the lumbar spine were tested by manual palpation. Spinal columns in the 12 week group were also subjected to non-destructive flexibility testing. MicroCT and histology were performed on a subset of each implant group at each euthanasia period.
Results: Radiographic scoring of the fusion sites indicated a normal healing response in all test groups. Bilateral radiographic fusion rates for all test groups were 0% at 4 weeks; ICBG 43%, Mastergraft Putty 50%, RB-85-0.2g 0%, RB-85-0.3g 13%, RB-85-0.45g 38%, and RB-85-0.6g 63% at 8 weeks; and ICBG 50%, Mastergraft Putty 50%, RB-85-0.2g 0%, RB-85-0.3g 25%, RB-85-0.45g 36%, and RB-85-0.6g 50% at 12 weeks.Spine fusion was assessed by manual palpation of the treated motion segments. At 12 weeks, ICBG, MGP, and RB-85-0.6g were fused mechanically in at least 50% of the rabbits. All groups demonstrated significantly less range of motion in both flexion/extension, lateral bending, and axial rotation compared to normal unfused controls.Histopathology analysis of the fusion masses, in all test groups, indicated an expected normal response of mild inflammation with macrophage and multinucleated giant cell response to the graft material at 4 weeks and resolving by 12 weeks. Regardless of test article, new bone formation and graft resorption increased from 4 to 12 weeks post-op.
Conclusions: This animal study has demonstrated the biocompatibility and normal healing features associated with the ReBOSSIS-85 bone graft (test material) when combined with autograft as an extender. ReBOSSIS-85 was more effective when a larger mass of test article was used in this study.
Clinical relevance: ReBOSSIS-85 can be used as an extender negating the need for large amounts of local or iliac crest bone in posterolateral fusions.
Keywords: animal model; posterolateral fusion; synthetic bone graft substitute.
Copyright © The Iowa Orthopaedic Journal 2019.
Conflict of interest statement
Disclosures: The authors report no potential conflicts of interest related to this study.
Figures
Similar articles
-
Influence of 45S5 Bioactive Glass in A Standard Calcium Phosphate Collagen Bone Graft Substitute on the Posterolateral Fusion of Rabbit Spine.Iowa Orthop J. 2017;37:193-198. Iowa Orthop J. 2017. PMID: 28852357 Free PMC article.
-
The efficacy of a nanosynthetic bone graft substitute as a bone graft extender in rabbit posterolateral fusion.Spine J. 2021 Nov;21(11):1925-1937. doi: 10.1016/j.spinee.2021.05.017. Epub 2021 May 23. Spine J. 2021. PMID: 34033931
-
Evaluation of a novel silicate substituted hydroxyapatite bone graft substitute in a rabbit posterolateral fusion model.Iowa Orthop J. 2013;33:25-32. Iowa Orthop J. 2013. PMID: 24027457 Free PMC article.
-
Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel.Spine (Phila Pa 1976). 1998 Jan 15;23(2):159-67. doi: 10.1097/00007632-199801150-00003. Spine (Phila Pa 1976). 1998. PMID: 9474720 Review.
-
Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: bone graft extenders and substitutes as an adjunct for lumbar fusion.J Neurosurg Spine. 2014 Jul;21(1):106-32. doi: 10.3171/2014.4.SPINE14325. J Neurosurg Spine. 2014. PMID: 24980593 Review.
Cited by
-
Designing electrospun fiber platforms for efficient delivery of genetic material and genome editing tools.Adv Drug Deliv Rev. 2022 Apr;183:114161. doi: 10.1016/j.addr.2022.114161. Epub 2022 Feb 17. Adv Drug Deliv Rev. 2022. PMID: 35183657 Free PMC article. Review.
-
An evaluation of novel AMP2-coated electrospun composite scaffolds for intraoral bone regeneration: a proof-of-concept in vivo study.Front Bioeng Biotechnol. 2025 Apr 17;13:1443280. doi: 10.3389/fbioe.2025.1443280. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40313641 Free PMC article.
-
Endodontic Regeneration Therapy: Current Strategies and Tissue Engineering Solutions.Cells. 2025 Mar 12;14(6):422. doi: 10.3390/cells14060422. Cells. 2025. PMID: 40136671 Free PMC article. Review.
-
Efficacy and Safety of Beta-Tricalcium Phosphate/Polylactic-Co-Glycolic Acid for Implantation of Bone Defects.Cureus. 2023 Aug 16;15(8):e43597. doi: 10.7759/cureus.43597. eCollection 2023 Aug. Cureus. 2023. PMID: 37719590 Free PMC article.
-
In Vivo Evaluation of Bone Regenerative Capacity of the Novel Nanobiomaterial: β-Tricalcium Phosphate Polylactic Acid-co-Glycolide (β-TCP/PLLA/PGA) for Use in Maxillofacial Bone Defects.Nanomaterials (Basel). 2023 Dec 28;14(1):91. doi: 10.3390/nano14010091. Nanomaterials (Basel). 2023. PMID: 38202548 Free PMC article.
References
-
- Arrington ED, Smith WJ, Chambers HG, et al. Complications of iliac crest bone graft harvesting. Clin.Orthop.Relat Res. 1996. pp. 300–9. - PubMed
-
- Xu R, Ebraheim NA, Yeasting RA, Jackson WT. Anatomic considerations for posterior iliac bone harvesting. Spine. 1996;21(9):1017–20. - PubMed
-
- Heppenstall B. Surgery of the Musculoskeletal System. New York: Churchill Livingston; 1983. Bone grafting; pp. 89–106. In: McCollster Evarts C, ed.
-
- Chase SW, Herndon CH: The fate of autogenous and homogenous bone grafts: A historical review. J Bone Joint Surg. 1955;37A:809–841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous