A simple method for long-term vital-staining of ciliated epidermal cells in aquatic larvae
- PMID: 32577422
- PMCID: PMC7300427
- DOI: 10.14440/jbm.2020.320
A simple method for long-term vital-staining of ciliated epidermal cells in aquatic larvae
Abstract
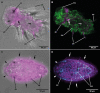
Observing the process of growth and differentiation of tissues and organs is of crucial importance for the understanding of the evolution of organs in animals. Unfortunately, it is notoriously difficult to continuously monitor developmental processes due to the extended time they take. Long-term labeling of the tissues of interest represents a promising alternative to raise these pivotal data. In the case of the prototroch, a band of ciliated cells typical of marine, planktotrophic trochophora larvae, we were able to apply a long-term fluorescent vital-staining to the prototroch cells that remains detectable throughout further larval life. We were able to stain ciliated cells of planktonic larvae from different spiralian clades by using long-chain dialkylcarbocyanine dyes that are detectable in different fluorescent emission spectra in combination with a non-ionic surfactant. The larvae survived and developed normally, their ciliated cells retaining the originally applied fluorescent labels. Combined with additional fluorescent staining of the larvae after fixation, we provide an easy, versatile, and broadly applicable method to investigate the processes of the differentiation of epidermal organs in various aquatic larvae.
Keywords: DiI; Spiralia; cLSM; fluorescent staining; prototroch.
© 2013-2020 The Journal of Biological Methods, All rights reserved.
Conflict of interest statement
Competing interests: The authors have declared that no competing interests exist.
Figures


References
LinkOut - more resources
Full Text Sources
