A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques
- PMID: 32577525
- PMCID: PMC7286676
- DOI: 10.1126/sciadv.aba8399
A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques
Abstract
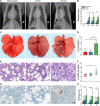
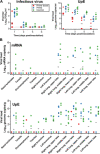
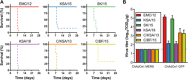
Developing a vaccine to protect against the lethal effects of the many strains of coronavirus is critical given the current global pandemic. For Middle East respiratory syndrome coronavirus (MERS-CoV), we show that rhesus macaques seroconverted rapidly after a single intramuscular vaccination with ChAdOx1 MERS. The vaccine protected against respiratory injury and pneumonia and reduced viral load in lung tissue by several orders of magnitude. MERS-CoV replication in type I and II pneumocytes of ChAdOx1 MERS-vaccinated animals was absent. A prime-boost regimen of ChAdOx1 MERS boosted antibody titers, and viral replication was completely absent from the respiratory tract tissue of these rhesus macaques. We also found that antibodies elicited by ChAdOx1 MERS in rhesus macaques neutralized six different MERS-CoV strains. Transgenic human dipeptidyl peptidase 4 mice vaccinated with ChAdOx1 MERS were completely protected against disease and lethality for all different MERS-CoV strains. The data support further clinical development of ChAdOx1 MERS.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- WHO, Middle East Respiratory Syndrome (2019); www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html.
-
- Bin S. Y., Heo J. Y., Song M.-S., Lee J., Kim E.-H., Park S.-J., Kwon H.-i., Kim S. M., Kim Y.-i., Si Y.-J., Lee I.-W., Baek Y. H., Choi W.-S., Min J., Jeong H. W., Choi Y. K., Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in south korea. Clin. Infect. Dis. 62, 755–760 (2016). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

