Enhancing radiation response by a second-generation TRAIL receptor agonist using a new in vitro organoid model system
- PMID: 32577539
- PMCID: PMC7303921
- DOI: 10.1016/j.ctro.2020.05.012
Enhancing radiation response by a second-generation TRAIL receptor agonist using a new in vitro organoid model system
Abstract
Background: For many cancer types, including colorectal carcinoma (CRC), combined modality treatments have shown to improve outcome, but are frequently associated with significant toxicity, illustrating the need for new therapeutic approaches. Based on preclinical data, TRAIL receptor agonists appeared to be promising agents for cancer therapy especially in combination with DNA damaging regimens. Here, we present the combination of the second-generation TRAIL receptor agonist APG-880 with radiation in a new and clinically relevant 3D model system.
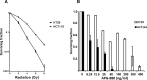
Methods: To investigate the effect of APG-880 in combination with radiation we performed short-term cytotoxicity and long-term clonogenic survival assays in established CRC cell lines, and in tumor organoids derived from colon cancer patients.
Results: APG-880 is a potent inducer of apoptosis in CRC cell lines and in patient-derived CRC organoids. Furthermore, a supra-additive effect on cytotoxicity was found when APG-880 and radiation were combined simultaneously, with combination indices around 0.7. Lastly, in the long-term survival assays, we demonstrated a radiosensitizing effect of APG-880 with dose enhancement factors between 1.3 and 1.5.
Conclusions: In a new, clinically relevant CRC-organoid model system we demonstrated a more than additive combined effect between the second-generation TRAIL receptor agonist APG-880 and radiation.
Keywords: Clonogenic survival; Colorectal carcinoma; Organoids; Radiation; TRAIL receptor agonist.
© 2020 The Author(s).
Conflict of interest statement
This work was partially supported by a research grant from AbbVie Inc. The funding source had no role in the study design. AbbVie Inc. participated in the interpretation of data, review, and approval of this publication.
Figures







References
-
- Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. - PubMed
-
- Swellengrebel H.A.M. Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br J Surg. 2011;98(3):418–426. - PubMed
-
- Brunner T.B. The rationale of combined radiotherapy and chemotherapy – joint action of Castor and Pollux. Best Pract Res Clin Gastroenterol. 2016;30(4):515–528. - PubMed
LinkOut - more resources
Full Text Sources

