This is a preprint.
Tocilizumab for treatment of mechanically ventilated patients with COVID-19
- PMID: 32577684
- PMCID: PMC7302290
- DOI: 10.1101/2020.05.29.20117358
Tocilizumab for treatment of mechanically ventilated patients with COVID-19
Update in
-
Tocilizumab for Treatment of Mechanically Ventilated Patients With COVID-19.Clin Infect Dis. 2021 Jul 15;73(2):e445-e454. doi: 10.1093/cid/ciaa954. Clin Infect Dis. 2021. PMID: 32651997 Free PMC article.
Abstract
Background: Severe COVID-19 can manifest in rapid decompensation and respiratory failure with elevated inflammatory markers. This presentation is consistent with cytokine release syndrome in chimeric antigen receptor T cell therapy, for which IL-6 blockade is approved treatment.
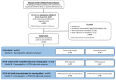
Methods: We assessed effectiveness and safety of IL-6 blockade with tocilizumab in a single-center cohort of patients with COVID-19 requiring mechanical ventilation. The primary endpoint was survival probability post-intubation; secondary analyses included an ordinal illness severity scale integrating superinfections. Outcomes in patients who received tocilizumab compared to tocilizumab-untreated controls were evaluated using multivariable Cox regression with propensity score inverse probability weighting (IPTW).
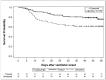
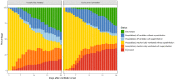
Findings: 154 patients were included, of whom 78 received tocilizumab and 76 did not. Median follow-up was 47 days (range 28-67). Baseline characteristics were similar between groups, although tocilizumab-treated patients were younger (mean 55 vs. 60 years), less likely to have chronic pulmonary disease (10% vs. 28%), and had lower D-dimer values at time of intubation (median 2.4 vs. 6.5 mg/dL). In IPTW-adjusted models, tocilizumab was associated with a 45% reduction in hazard of death [hazard ratio 0.55 (95% CI 0.33, 0.90)] and improved status on the ordinal outcome scale [odds ratio per 1-level increase: 0.59 (0.36, 0.95)]. Though tocilizumab was associated with an increased proportion of patients with superinfections (54% vs. 26%; p<0.001), there was no difference in 28-day case fatality rate among tocilizumab-treated patients with versus without superinfection [22% vs. 15%; p=0.42].
Interpretation: In this cohort of mechanically ventilated COVID-19 patients, tocilizumab was associated with a decreased likelihood of death despite higher superinfection occurrence. Randomized controlled trials are urgently needed to confirm these findings.
Keywords: COVID-19; SARS-CoV-2; interleukin-6; tocilizumab.
Figures

References
-
- Petrilli CM, Jones SA, Yang J, et al. Title: Factors associated with hospitalization and critical illness among 4,103 patients with. PREPRINT. 2020:https://www.medrxiv.org/content/10.1101/2020.04.08. doi:10.1101/2020.04.08.20057794 - DOI - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
