Ripretinib: First Approval
- PMID: 32578014
- PMCID: PMC7595980
- DOI: 10.1007/s40265-020-01348-2
Ripretinib: First Approval
Erratum in
-
Correction to: Ripretinib: First Approval.Drugs. 2020 Dec;80(18):1999. doi: 10.1007/s40265-020-01430-9. Drugs. 2020. PMID: 33156461 Free PMC article.
Abstract
Ripretinib (QINLOCK™) is a novel type II tyrosine switch control inhibitor being developed by Deciphera Pharmaceuticals for the treatment of KIT proto-oncogene receptor tyrosine kinase (KIT)-driven and/or platelet derived growth factor receptor A (PDGFRA)-driven cancers, including gastrointestinal stromal tumour (GIST). Ripretinib inhibits KIT and PDGFRA kinase, including wild-type, primary and secondary mutations, as well as other kinases, such as PDGFRB, TIE2, VEGFR2 and BRAF. In May 2020, oral ripretinib received its first approval in the USA for the treatment of adult patients with advanced GIST who have received prior treatment with ≥ 3 kinase inhibitors, including imatinib. The US FDA, Health Canada and the Australian Therapeutic Goods Administration collaborated on the review of the ripretinib new drug application in this indication as part of Project Orbis; regulatory review in Australia and Canada is ongoing. Clinical development for GIST, solid tumours and systemic mastocytosis is underway in several countries worldwide. This article summarizes the milestones in the development of ripretinib leading to this first approval for the treatment of advanced GIST.
Conflict of interest statement
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Figures
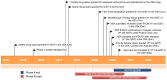
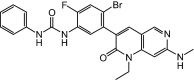
References
-
- National Comprehensive Cancer Institute. Gastroinestinal Stromal Tumors (GIST): NCCN gudelines Version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf. Accessed 26 May 2020.
-
- US Food & Drug Administration. FDA approves ripretinib for advanced gastrointestinal stromal tumor [media release] 15 May 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-ripr....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

