Handling and performance characteristics of a new small caliber radiopaque embolic microsphere
- PMID: 32578348
- PMCID: PMC7496950
- DOI: 10.1002/jbm.b.34619
Handling and performance characteristics of a new small caliber radiopaque embolic microsphere
Abstract
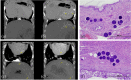
The in vitro and in vivo handling and performance characteristics of a small caliber radiopaque embolic microsphere, 40-90 μm DC Bead LUMI™ (LUMI40-90), were studied. Microsphere drug loading and elution and effects on size, suspension, and microcatheter delivery were evaluated using established in vitro methodologies. In vivo evaluations of vascular penetration (rabbit renal artery embolization), long-term biocompatibility and X-ray imaging properties, pharmacokinetics and local tissue effects of both doxorubicin (Dox) and irinotecan (Iri) loaded microspheres (swine hepatic artery embolization) were conducted. Compared to 70-150 μm DC Bead LUMI (LUMI70-150), LUMI40-90 averaged 70 μm versus 100 μm, which was unchanged upon drug loading. Handling, suspension, and microsphere delivery studies were successfully performed. Dox loading was faster (20 min) and Iri equivalent (<10 min) while drug elution rates were similar. Contrast suspension times were longer with no delivery complications. Vascular penetration was statistically greater (rabbit) with no unexpected adverse safety findings (swine). Microspheres ± drug were visible under X-ray imaging (CT) at 90 days. Peak plasma drug levels and area under the curve were greater for LUMI40-90 compared to LUMI70-150 but comparable to 70-150 μm DC BeadM1™ (DC70-150). Local tissue effects showed extensive hepatic necrosis for Dox, whereas Iri displayed lower toxicity with more pronounced lobar fibrosis. LUMI40-90 remains suspended for longer and have greater vessel penetration compared to the other DC Bead LUMI sizes and are similarly highly biocompatible with long-term visibility under X-ray imaging. Drug loading is equivalent or faster with pharmacokinetics similar to DC70-150 for both Dox and Iri.
Keywords: DC Bead LUMI; X-ray imageability; biocompatibility; drug loading and elution; microsphere penetration; pharmacokinetics; suspension and delivery.
© 2020 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials published by Wiley Periodicals LLC.
Conflict of interest statement
All of the authors named on this manuscript are employees of Biocompatibles UK Ltd., the manufacturer of the test device that was the subject of this study. Some of these data were generated for use in filings with Regulatory agencies to demonstrate the safety of the device.
Figures





References
-
- Aliberti, C. , Carandina, R. , Sarti, D. , Pizzirani, E. , Ramondo, G. , Cillo, U. , … Fiorentini, G. (2017). Transarterial chemoembolization with DC bead LUMI radiopaque beads for primary liver cancer treatment: Preliminary experience. Future Oncology, 13(25), 2243–2252. - PubMed
-
- Beaujeux, R. , Laurent, A. , Wassef, M. , Casasco, A. , Gobin, Y. P. , Aymard, A. , … Merland, J. J. (1996). Trisacryl gelatin microspheres for therapeutic embolization, II: Preliminary clinical evaluation in tumors and arteriovenous malformations. American Journal of Neuroradiology, 17(3), 541–548. - PMC - PubMed
-
- Bonomo, G. , Pedicini, V. , Monfardini, L. , Della Vigna, P. , Poretti, D. , Orgera, G. , & Orsi, F. (2010). Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size‐calibrated, anti‐inflammatory microparticles: First clinical experience and one‐year follow‐up. Cardiovascular and Interventional Radiology, 33(3), 552–559. - PubMed
-
- Brown, K. T. (2004). Fatal pulmonary complications after arterial embolization with 40‐120‐ micro m tris‐acryl gelatin microspheres. Journal of Vascular and Interventional Radiology, 15(2 Pt 1), 197–200. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

