Dosage of heparin for patency of the totally implanted central venous catheter in cancer patients
- PMID: 32578754
- PMCID: PMC7304977
- DOI: 10.1590/1518-8345.3326.3304
Dosage of heparin for patency of the totally implanted central venous catheter in cancer patients
Abstract
Objective: to analyze the evidence available in the literature about the lowest necessary dose of heparin to maintain the patency of the totally implanted central venous catheter in adult cancer patients.
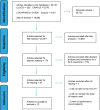
Method: an integrative literature review, carried out in the following databases: Literatura Latino-Americana e do Caribe em Ciências de Saúde, Sciverse Scopus, Web of Science, Cumulative Index to Nursing and Allied Health Literature, Cochrane Central Register of Controlled Trials, including thirteen studies.
Results: the evidence showed that the dose of heparin (300 IU/ml) is the most used in maintaining the patency of the totally implanted central venous catheter.
Conclusion: according to the selected studies, the lowest dose of heparin found in maintaining the patency of the totally implanted central venous catheter in cancer patients was 10 UN/ml with a volume of 5 ml of the heparin solution.
Objetivo:: analisar as evidências disponíveis na literatura sobre a menor dose necessária de heparina para manter a patência do cateter venoso central totalmente implantado em pacientes oncológicos adultos.
Método:: revisão integrativa da literatura, realizada nas bases de dados: Literatura Latino-Americana e do Caribe em Ciências de Saúde, Sciverse SCOPUS, Web of Science, Cumulative Index to Nursing and Allied Health Literature, Cochrane Central Register of Controlled Trials, sendo incluídos treze estudos.
Resultados:: as evidências mostraram que a dose de heparina (300 UI/ml), é a mais utilizada na manutenção da patência do cateter venoso central totalmente implantado.
Conclusão:: de acordo com os estudos selecionados a menor dose de heparina encontrada na manutenção da patência do cateter venoso central totalmente implantado em pacientes oncológicos, foi de 10 UN/ml com um volume de 5 ml da solução de heparina.
Objetivo:: analizar la evidencia disponible en la literatura sobre la dosis más baja de heparina necesaria para mantener la permeabilidad del catéter venoso central totalmente implantado en pacientes oncológicos adultos.
Método:: revisión integradora de la literatura realizada en las siguientes bases de datos: Literatura Latinoamericana y del Caribe en Ciencias de la Salud, Sciverse Scopus, Web of Science, Cumulative Index to Nursing and Allied Health Literature, Cochrane Central Register of Controlled Trials, con la inclusión de trece estudios.
Resultados:: las pruebas demostraron que la dosis de heparina (300 UI/ml) es la más utilizada para mantener la permeabilidad del catéter venoso central totalmente implantado.
Conclusión:: según los estudios seleccionados, la dosis más baja de heparina encontrada en el mantenimiento de la permeabilidad del catéter venoso central totalmente implantado en pacientes oncológicos fue de 10 UN/ml con un volumen de 5 ml de solución de heparina.
Figures
References
-
- Girda E, Phaeton R, Goldberg GL, Kuo D. Extending the interval for port-a-cath maintenance. Modern Chemother. 2013 Oct;2(2):15–18. doi: 10.4236/mc.2013.22003. - DOI
-
- Palese A, Baldassar D, Rupil A, Bonanni G, Capellari Maria T, Contessi D, et al. Maintaining patency in totally implantable venous access devices (TIVAD): a time-to-event analysis of different lock irrigation intervals. Eur J Oncol Nurs. 2014 Feb;18(1):66–71. doi: 10.1016/j.ejon.2013.09.002. - DOI - PubMed
-
- Infusion Nurses Society Infusion nursing standards of practice. [Sept 10, 2018];J Infus Nurs. 2016 Jan-Feb;39(1S) [Internet] Available from: http://source.yiboshi.com/20170417/1492425631944540325.pdf.
-
- Hill J, Broadhurst D, Miller K, Cook C, Dumanski J, Friesen N, et al. Occlusion management guideline for central venous access devices (CVADs) [Sept 10, 2018];Vasc Access. 2013 7(Supplement 1) [Internet] Available from: http://www.improvepicc.com/uploads/5/6/5/0/56503399/omg_2013_final_revis....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

