A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial
- PMID: 32578850
- PMCID: PMC8202417
- DOI: 10.1093/eurheartj/ehaa419
A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial
Abstract
Aim: We tested the hypothesis that dapagliflozin may regress left ventricular hypertrophy (LVH) in people with type 2 diabetes (T2D).
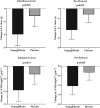
Methods and results: We randomly assigned 66 people (mean age 67 ± 7 years, 38 males) with T2D, LVH, and controlled blood pressure (BP) to receive dapagliflozin 10 mg once daily or placebo for 12 months. Primary endpoint was change in absolute left ventricular mass (LVM), assessed by cardiac magnetic resonance imaging. In the intention-to-treat analysis, dapagliflozin significantly reduced LVM compared with placebo with an absolute mean change of -2.82g [95% confidence interval (CI): -5.13 to -0.51, P = 0.018]. Additional sensitivity analysis adjusting for baseline LVM, baseline BP, weight, and systolic BP change showed the LVM change to remain statistically significant (mean change -2.92g; 95% CI: -5.45 to -0.38, P = 0.025). Dapagliflozin significantly reduced pre-specified secondary endpoints including ambulatory 24-h systolic BP (P = 0.012), nocturnal systolic BP (P = 0.017), body weight (P < 0.001), visceral adipose tissue (VAT) (P < 0.001), subcutaneous adipose tissue (SCAT) (P = 0.001), insulin resistance, Homeostatic Model Assessment of Insulin Resistance (P = 0.017), and high-sensitivity C-reactive protein (hsCRP) (P = 0.049).
Conclusion: Dapagliflozin treatment significantly reduced LVM in people with T2D and LVH. This reduction in LVM was accompanied by reductions in systolic BP, body weight, visceral and SCAT, insulin resistance, and hsCRP. The regression of LVM suggests dapagliflozin can initiate reverse remodelling and changes in left ventricular structure that may partly contribute to the cardio-protective effects of dapagliflozin.
Clinicaltrials.gov identifier: NCT02956811.
Keywords: Dapagliflozin; Heart failure; Insulin resistance; Left ventricular mass; Type 2 diabetes.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
Comment in
-
Regression of left ventricular hypertrophy with SGLT2 inhibitors.Eur Heart J. 2020 Sep 21;41(36):3433-3436. doi: 10.1093/eurheartj/ehaa530. Eur Heart J. 2020. PMID: 32620974 No abstract available.
-
Dapagliflozin reduces left ventricular mass.Nat Rev Cardiol. 2020 Sep;17(9):540. doi: 10.1038/s41569-020-0417-5. Nat Rev Cardiol. 2020. PMID: 32632233 No abstract available.
-
Dapagliflozin Improves Left Ventricular Myocardial Longitudinal Function in Patients With Type 2 Diabetes.JACC Cardiovasc Imaging. 2021 Feb;14(2):503-504. doi: 10.1016/j.jcmg.2020.07.025. Epub 2020 Sep 11. JACC Cardiovasc Imaging. 2021. PMID: 32928703 No abstract available.
References
-
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M.. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234. - PubMed
-
- Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, O’Keeffe LM, Gao P, Wood AM, Burgess S, Freitag DF, Pennells L, Peters SA, Hart CL, Haheim LL, Gillum RF, Nordestgaard BG, Psaty BM, Yeap BB, Knuiman MW, Nietert PJ, Kauhanen J, Salonen JT, Kuller LH, Simons LA, van der Schouw YT, Barrett-Connor E, Selmer R, Crespo CJ, Rodriguez B, Verschuren WM, Salomaa V, Svardsudd K, van der Harst P, Bjorkelund C, Wilhelmsen L, Wallace RB, Brenner H, Amouyel P, Barr EL, Iso H, Onat A, Trevisan M, D’Agostino RB Sr, Cooper C, Kavousi M, Welin L, Roussel R, Hu FB, Sato S, Davidson KW, Howard BV, Leening MJ, Rosengren A, Dorr M, Deeg DJ, Kiechl S, Stehouwer CD, Nissinen A, Giampaoli S, Donfrancesco C, Kromhout D, Price JF, Peters A, Meade TW, Casiglia E, Lawlor DA, Gallacher J, Nagel D, Franco OH, Assmann G, Dagenais GR, Jukema JW, Sundstrom J, Woodward M, Brunner EJ, Khaw KT, Wareham NJ, Whitsel EA, Njolstad I, Hedblad B, Wassertheil-Smoller S, Engstrom G, Rosamond WD, Selvin E, Sattar N, Thompson SG, Danesh J.. Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60. - PMC - PubMed
-
- Kannel WB, Hjortland M, Castelli WP.. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34:29–34. - PubMed
-
- Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC Jr, Grimm RH Jr, Margolis KL, Probstfield JL,, Simons-Morton DG, Sullivan MD.. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99:21i–33i. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials