Fibroblast growth factor 23: a biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction
- PMID: 32578967
- PMCID: PMC7524237
- DOI: 10.1002/ehf2.12816
Fibroblast growth factor 23: a biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction
Abstract
Aims: Besides regulating calcium-phosphate metabolism, fibroblast growth factor 23 (FGF-23) has been associated with incident heart failure (HF) and left ventricular hypertrophy. However, data about FGF-23 in HF and preserved ejection fraction (HFpEF) remain limited. The aim of this study was to assess the association between FGF-23 levels, clinical and imaging characteristics, particularly diffuse myocardial fibrosis, and prognosis in HFpEF patients.
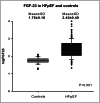
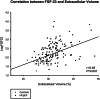
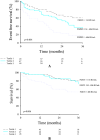
Methods and results: We prospectively included 143 consecutive HFpEF patients (78 ± 8 years, 61% female patients) and 31 controls of similar age and gender (75 ± 6 years, 61% female patients). All subjects underwent a complete two-dimensional echocardiography and cardiac magnetic resonance with extracellular volume (ECV) assessment by T1 mapping. FGF-23 was measured at baseline. Among the patients, differences in clinical and imaging characteristics across tertiles of FGF-23 levels were analysed with a trend test across the ordered groups. Patients were followed over time for a primary endpoint of all-cause mortality and first HF hospitalization and a secondary endpoint of all-cause mortality. Median FGF-23 was significantly higher in HFpEF patients compared with controls of similar age and gender (247 [115; 548] RU/mL vs. 61 [51; 68] RU/mL, P < 0.001). Among HFpEF patients, higher FGF-23 levels were associated with female sex, higher incidence of atrial fibrillation, lower haemoglobin, worse renal function, and higher N terminal pro brain natriuretic peptide levels (P for trend < 0.05 for all). Regarding imaging characteristics, patients with higher FGF-23 levels had greater left atrial volumes, worse right ventricular systolic function, and more fibrosis estimated by ECV (P for trend < 0.05 for all). FGF-23 was moderately correlated with ECV (r = 0.46, P < 0.001). Over a mean follow-up of 30 ± 8 months, 43 patients (31%) died and 69 patients (49%) were hospitalized for HF. A total of 87 patients (62%) reached the primary composite endpoint of all-cause mortality and/or first HF hospitalization. In multivariate Cox regression analysis for the primary endpoint, FGF-23 (HR: 3.44 [2.01; 5.90], P < 0.001) and E wave velocities (HR: 1.01 [1.00; 1.02], P = 0.034) were independent predictors of the primary composite endpoint. In multivariate Cox regression analysis for the secondary endpoint, ferritin (HR: 1.02 [1.01; 1.03], P < 0.001), FGF-23 (HR: 2.85 [1.26; 6.44], P = 0.012), and ECV (HR: 1.26 [1.03; 1.23], P = 0.008) were independent predictors of all-cause mortality.
Conclusions: Fibroblast growth factor 23 (FGF-23) levels were significantly higher in HFpEF patients compared with controls of similar age and gender. FGF-23 was correlated with fibrosis evaluated by ECV. High levels of FGF-23 were significantly associated with signs of disease severity such as worse renal function, larger left atrial volumes, and right ventricular dysfunction. Moreover, FGF-23 was a strong predictor of poor outcome (mortality and first HF hospitalization).
Keywords: Biomarker; FGF-23; Fibrosis; Heart failure; Mortality; NT-proBNP; Prognosis; Troponin.
© 2020 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of the European Society of Cardiology.
Conflict of interest statement
The Cliniques St. Luc UCL has a master clinical research agreement with Philips Medical Instruments, and the MOLLI patch was supplied by Philips Medical under the terms of this agreement. The authors declare that they have no competing interests.
Figures
References
-
- Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. - PubMed
-
- Duca F, Kammerlander AA, Zotter‐Tufaro C, Aschauer S, Schwaiger ML, Marzluf BA, Bonderman D, Mascherbauer J. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: insights from a prospective cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging 2016; 9e005277 - PubMed
-
- Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Taguri M, Kimura K, Umemura S. Prognostic significance of quantitative assessment of focal myocardial fibrosis in patients with heart failure with preserved ejection fraction. Int J Cardiol 2015; 191: 314–319. - PubMed
-
- Roy C, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, Beauloye C, Vanoverschelde JL, Gerber BL, Pouleur AC. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson 2018; 20: 55. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

