An autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through deregulated VEGFA, NOS1, and CTNNB1
- PMID: 32579471
- PMCID: PMC8354601
- DOI: 10.1080/15548627.2020.1778292
An autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through deregulated VEGFA, NOS1, and CTNNB1
Abstract
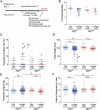
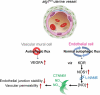
The uterus undergoes vascular changes during the reproductive cycle and pregnancy. Steroid hormone deprivation induces macroautophagy/autophagy in major uterine cell types. Herein, we explored the functions of uterine autophagy using the Amhr2-Cre-driven atg7 deletion model. Deletion of Atg7 was confirmed by functional deficit of autophagy in uterine stromal, myometrial, and vascular smooth muscle cells, but not in endothelial cells. atg7d/d uteri exhibited enhanced stromal edema accompanied by dilation of blood vessels. Ovariectomized atg7d/d uteri showed decreased expression of endothelial junction-related proteins, such as CTNNB1/beta-catenin, with increased vascular permeability, and increased expression of VEGFA and NOS1. Nitric oxide (NO) was shown to mediate VEGFA-induced vascular permeability by targeting CTNNB1. NO involvement in maintaining endothelial junctional stability in atg7d/d uteri was confirmed by the reduction in extravasation following treatment with a NOS inhibitor. We also showed that atg7d/d uterine phenotype improved the fetal weight:placental weight ratio, which is one of the indicators of assessing the status of preeclampsia. We showed that autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through the deregulation of VEGFA, NOS1, and CTNNB1.Abbreviations: ACTA2: actin, alpha 2, smooth muscle, aortic; Amhr2: anti-Mullerian hormone type 2 receptor; ANGPT1: angiopoietin 1; ATG: autophagy-related; CDH5: cadherin 5; CLDN5: claudin 5; COL1A1: collagen, type I, alpha 1; CSPG4/NG2: chondroitin sulfate proteoglycan 4; CTNNB1: catenin (cadherin associated protein), beta 1; DES: desmin; EDN1: endothelin 1; EDNRB: endothelin receptor type B; F3: coagulation factor III; KDR/FLK1/VEGFR2: kinase insert domain protein receptor; LYVE1: lymphatic vessel endothelial hyaluronan receptor 1; MAP1LC3B: microtubule-associated protein 1 light chain 3 beta; MCAM/CD146: melanoma cell adhesion molecule; MYL2: myosin, light polypeptide 2, regulatory, cardiac, slow; MYLK: myosin, light polypeptide kinase; NOS1/nNOS: nitric oxide synthase 1, neuronal; NOS2/iNOS: nitric oxide synthase 2, inducible; NOS3/eNOS: nitric oxide synthase 3, endothelial cell; OVX: ovariectomy; PECAM1/CD31: platelet/endothelial cell adhesion molecule 1; POSTN: periostin, osteoblast specific factor; SQSTM1: sequestosome 1; TEK/Tie2: TEK receptor tyrosine kinase; TJP1/ZO-1: tight junction protein 1; TUBB1, tubulin, beta 1 class VI; USC: uterine stromal cell; VEGFA: vascular endothelial growth factor A; VSMC: vascular smooth muscle cell.
Keywords: Atg7; autophagy; permeability; uterus; vascular factor.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

References
-
- Mizushima N.Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. - PubMed
-
- Tanida I, Tanida-Miyake E, Ueno T, et al. The human homolog of Saccharomyces cerevisiae Apg7p is a Protein-activating enzyme for multiple substrates including human Apg12p, GATE-16, GABARAP, and MAP-LC3. J Biol Chem. 2001;276(3):1701–1706. - PubMed
-
- Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
