Serum Urate Lowering with Allopurinol and Kidney Function in Type 1 Diabetes
- PMID: 32579810
- PMCID: PMC7375708
- DOI: 10.1056/NEJMoa1916624
Serum Urate Lowering with Allopurinol and Kidney Function in Type 1 Diabetes
Abstract
Background: Higher serum urate levels are associated with an increased risk of diabetic kidney disease. Lowering of the serum urate level with allopurinol may slow the decrease in the glomerular filtration rate (GFR) in persons with type 1 diabetes and early-to-moderate diabetic kidney disease.
Methods: In a double-blind trial, we randomly assigned participants with type 1 diabetes, a serum urate level of at least 4.5 mg per deciliter, an estimated GFR of 40.0 to 99.9 ml per minute per 1.73 m2 of body-surface area, and evidence of diabetic kidney disease to receive allopurinol or placebo. The primary outcome was the baseline-adjusted GFR, as measured with iohexol, after 3 years plus a 2-month washout period. Secondary outcomes included the decrease in the iohexol-based GFR per year and the urinary albumin excretion rate after washout. Safety was also assessed.
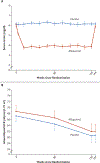
Results: A total of 267 patients were assigned to receive allopurinol and 263 to receive placebo. The mean age was 51.1 years, the mean duration of diabetes 34.6 years, and the mean glycated hemoglobin level 8.2%. The mean baseline iohexol-based GFR was 68.7 ml per minute per 1.73 m2 in the allopurinol group and 67.3 ml per minute per 1.73 m2 in the placebo group. During the intervention period, the mean serum urate level decreased from 6.1 to 3.9 mg per deciliter with allopurinol and remained at 6.1 mg per deciliter with placebo. After washout, the between-group difference in the mean iohexol-based GFR was 0.001 ml per minute per 1.73 m2 (95% confidence interval [CI], -1.9 to 1.9; P = 0.99). The mean decrease in the iohexol-based GFR was -3.0 ml per minute per 1.73 m2 per year with allopurinol and -2.5 ml per minute per 1.73 m2 per year with placebo (between-group difference, -0.6 ml per minute per 1.73 m2 per year; 95% CI, -1.5 to 0.4). The mean urinary albumin excretion rate after washout was 40% (95% CI, 0 to 80) higher with allopurinol than with placebo. The frequency of serious adverse events was similar in the two groups.
Conclusions: We found no evidence of clinically meaningful benefits of serum urate reduction with allopurinol on kidney outcomes among patients with type 1 diabetes and early-to-moderate diabetic kidney disease. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; PERL ClinicalTrials.gov number, NCT02017171.).
Copyright © 2020 Massachusetts Medical Society.
Figures

Comment in
-
Urate-Lowering Therapy and Chronic Kidney Disease Progression.N Engl J Med. 2020 Jun 25;382(26):2567-2568. doi: 10.1056/NEJMe2015886. N Engl J Med. 2020. PMID: 32579818 No abstract available.
-
Allopurinol: Good for Gout But Not for Preventing Loss of Kidney Function.Am J Kidney Dis. 2021 Mar;77(3):459-461. doi: 10.1053/j.ajkd.2020.09.001. Epub 2020 Sep 10. Am J Kidney Dis. 2021. PMID: 32920152 No abstract available.
-
Research in brief: Serum urate reduction and its effect on the progression of chronic kidney disease.Clin Med (Lond). 2020 Sep;20(5):448. doi: 10.7861/clinmed.rib.20.5.1. Clin Med (Lond). 2020. PMID: 32934035 Free PMC article. No abstract available.
-
Allopurinol and Chronic Kidney Disease.N Engl J Med. 2020 Oct 22;383(17):1687-1688. doi: 10.1056/NEJMc2026125. N Engl J Med. 2020. PMID: 33085871 No abstract available.
-
Allopurinol and Chronic Kidney Disease.N Engl J Med. 2020 Oct 22;383(17):1688-1689. doi: 10.1056/NEJMc2026125. N Engl J Med. 2020. PMID: 33085872 No abstract available.
-
Allopurinol and Chronic Kidney Disease.N Engl J Med. 2020 Oct 22;383(17):1689. doi: 10.1056/NEJMc2026125. N Engl J Med. 2020. PMID: 33085873 No abstract available.
-
Allopurinol and Chronic Kidney Disease.N Engl J Med. 2020 Oct 22;383(17):1689-1690. doi: 10.1056/NEJMc2026125. N Engl J Med. 2020. PMID: 33085874 No abstract available.
-
Allopurinol and Chronic Kidney Disease.N Engl J Med. 2020 Oct 22;383(17):1690. doi: 10.1056/NEJMc2026125. N Engl J Med. 2020. PMID: 33085875 No abstract available.
-
Hyperuricemia and progression of chronic kidney disease: to treat or not to treat?Kidney Int. 2021 Jan;99(1):14-16. doi: 10.1016/j.kint.2020.10.022. Kidney Int. 2021. PMID: 33390225 No abstract available.
References
-
- Leslie RD. United Kingdom Prospective Diabetes Study (UKPDS): what now or so what? Diabetes Metab Res Rev 1999; 15:65–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR002494/TR/NCATS NIH HHS/United States
- UC4 DK101108/DK/NIDDK NIH HHS/United States
- P30 AG024824/AG/NIA NIH HHS/United States
- UL1 TR002556/TR/NCATS NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- P30 AG08808/AG/NIA NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- R34 DK097808/DK/NIDDK NIH HHS/United States
- P30 DK020541/DK/NIDDK NIH HHS/United States
- 17-2012-377/Juvenile Diabetes Research Foundation United States of America/International
- UL1 TR002319/TR/NCATS NIH HHS/United States
- R03 DK094484/DK/NIDDK NIH HHS/United States
- UC4 D101108/DK/NIDDK NIH HHS/United States
- UL1 TR001105/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
