Nitazoxanide protects cats from feline calicivirus infection and acts synergistically with mizoribine in vitro
- PMID: 32579897
- PMCID: PMC7306210
- DOI: 10.1016/j.antiviral.2020.104827
Nitazoxanide protects cats from feline calicivirus infection and acts synergistically with mizoribine in vitro
Abstract
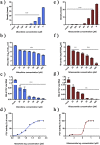
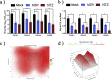
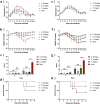
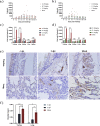
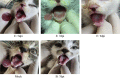
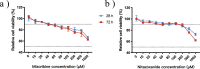
Feline calicivirus (FCV) is a highly contagious pathogen that causes acute upper respiratory infections and oral disease in cats, thus seriously endangering feline health. Recently, there have been outbreaks of particularly virulent variant strains of FCV, which can cause both acute symptoms and fatal systemic disease. The discovery of effective antiviral agents to treat FCV infection is, therefore, gradually assuming increased importance. In this study, we showed that both nitazoxanide and mizoribine had antiviral activity in F81 cells infected with different strains of FCV and also demonstrated a synergistic effect between the two drugs. Experiments in cats challenged with FCV showed that nitazoxanide significantly reduced the clinical symptoms of FCV infection, reduced viral load in the trachea and lungs, and reduced viral shedding. Our results showed that nitazoxanide and mizoribine could potentially be used as therapeutic agents to treat FCV infection.
Keywords: Antiviral; Feline calicivirus; In vivo; Mizoribine; Nitazoxanide.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Potential Therapeutic Agents for Feline Calicivirus Infection.Viruses. 2018 Aug 16;10(8):433. doi: 10.3390/v10080433. Viruses. 2018. PMID: 30115859 Free PMC article.
-
Antiviral effect of lithium chloride on feline calicivirus in vitro.Arch Virol. 2015 Dec;160(12):2935-43. doi: 10.1007/s00705-015-2534-8. Epub 2015 Aug 5. Arch Virol. 2015. PMID: 26239340 Free PMC article.
-
Antiviral effect of copper chloride on feline calicivirus and synergy with ribavirin in vitro.BMC Vet Res. 2020 Jul 6;16(1):231. doi: 10.1186/s12917-020-02441-0. BMC Vet Res. 2020. PMID: 32631322 Free PMC article.
-
[Treatment of acute viral feline upper respiratory tract infections].Tierarztl Prax Ausg K Kleintiere Heimtiere. 2019 Apr;47(2):98-109. doi: 10.1055/a-0870-0801. Epub 2019 Apr 23. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2019. PMID: 31013527 Review. German.
-
Feline calicivirus.Vet Res. 2007 Mar-Apr;38(2):319-35. doi: 10.1051/vetres:2006056. Epub 2007 Feb 13. Vet Res. 2007. PMID: 17296159 Review.
Cited by
-
miRNA-328-3p regulates ZO-1 expression and inhibits PEDV proliferation via the PLC-β1-PKC pathway.PLoS One. 2025 Jan 3;20(1):e0316074. doi: 10.1371/journal.pone.0316074. eCollection 2025. PLoS One. 2025. PMID: 39752351 Free PMC article.
-
Decitabine Increases the Transcription of RIG-I Gene to Suppress the Replication of Feline Calicivirus and Canine Influenza Virus.Microorganisms. 2025 Jan 13;13(1):143. doi: 10.3390/microorganisms13010143. Microorganisms. 2025. PMID: 39858911 Free PMC article.
-
Update on feline calicivirus: viral evolution, pathogenesis, epidemiology, prevention and control.Front Microbiol. 2024 May 2;15:1388420. doi: 10.3389/fmicb.2024.1388420. eCollection 2024. Front Microbiol. 2024. PMID: 38756726 Free PMC article. Review.
-
High-throughput screening unveils nitazoxanide as a potent PRRSV inhibitor by targeting NMRAL1.Nat Commun. 2024 Jun 6;15(1):4813. doi: 10.1038/s41467-024-48807-y. Nat Commun. 2024. PMID: 38844461 Free PMC article.
-
Antiviral Activity of Nitazoxanide and Miltefosine Against FeHV-1 In Vitro.Vet Med Int. 2024 Oct 19;2024:8849561. doi: 10.1155/2024/8849561. eCollection 2024. Vet Med Int. 2024. PMID: 39464309 Free PMC article.
References
-
- Aboubakr H.A., Nauertz A., Luong N.T., Agrawal S., El-Sohaimy S.A.A., Youssef M.M., Goyal S.M. In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. J. Food Protect. 2016;79:1001–1012. doi: 10.4315/0362-028X.JFP-15-593. - DOI - PubMed
-
- Camero M., Buonavoglia D., Lucente M.S., Losurdo M., Crescenzo G., Trerotoli P., Casalino E., Martella V., Elia G., Tempesta M. Enhancement of the antiviral activity against caprine herpesvirus type 1 of Acyclovir in association with Mizoribine. Res. Vet. Sci. 2017;111:120–123. doi: 10.1016/j.rvsc.2017.02.012. - DOI - PubMed
-
- Caringella F., Elia G., Decaro N., Martella V., Lanave G., Varello K., Catella C., Diakoudi G., Carelli G., Colaianni M.L., Bo S., Buonavoglia C. Feline calicivirus infection in cats with virulent systemic disease, Italy. Res. Vet. Sci. 2019;124:46–51. doi: 10.1016/j.rvsc.2019.02.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

