Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma
- PMID: 32579974
- PMCID: PMC7391009
- DOI: 10.1016/j.cell.2020.05.039
Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma
Erratum in
-
Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma.Cell. 2020 Sep 17;182(6):1661-1662. doi: 10.1016/j.cell.2020.08.043. Cell. 2020. PMID: 32946785 Free PMC article. No abstract available.
Abstract
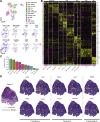
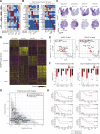
To define the cellular composition and architecture of cutaneous squamous cell carcinoma (cSCC), we combined single-cell RNA sequencing with spatial transcriptomics and multiplexed ion beam imaging from a series of human cSCCs and matched normal skin. cSCC exhibited four tumor subpopulations, three recapitulating normal epidermal states, and a tumor-specific keratinocyte (TSK) population unique to cancer, which localized to a fibrovascular niche. Integration of single-cell and spatial data mapped ligand-receptor networks to specific cell types, revealing TSK cells as a hub for intercellular communication. Multiple features of potential immunosuppression were observed, including T regulatory cell (Treg) co-localization with CD8 T cells in compartmentalized tumor stroma. Finally, single-cell characterization of human tumor xenografts and in vivo CRISPR screens identified essential roles for specific tumor subpopulation-enriched gene networks in tumorigenesis. These data define cSCC tumor and stromal cell subpopulations, the spatial niches where they interact, and the communicating gene networks that they engage in cancer.
Keywords: CRISPR screen; MIBI; intra-tumoral heterogeneity; multi-omics; scRNA-seq; skin cancer; spatial transcriptomics; squamous cell carcinoma; tumor immunology; tumor microenvironment.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests G.P.N. is a co-founder and stockholder of Ionpath and an inventor on patent US20150287578A1. J.L. is a scientific consultant for 10X Genomics.
Figures













References
-
- Browaeys R., Saelens W., Saeys Y. NicheNet: Modeling intercellular communication by linking ligands to target genes. Nat. Methods. 2019;17:159–162. - PubMed
Publication types
MeSH terms
Grants and funding
- 27145/CRUK_/Cancer Research UK/United Kingdom
- UG3 DK114937/DK/NIDDK NIH HHS/United States
- U19 AI100627/AI/NIAID NIH HHS/United States
- R01 HL120724/HL/NHLBI NIH HHS/United States
- U01 AI101984/AI/NIAID NIH HHS/United States
- U54 HG010426/HG/NHGRI NIH HHS/United States
- C27165/A29073/CRUK_/Cancer Research UK/United Kingdom
- F99 CA212231/CA/NCI NIH HHS/United States
- U01 AI140498/AI/NIAID NIH HHS/United States
- F32 CA233203/CA/NCI NIH HHS/United States
- R01 AR043799/AR/NIAMS NIH HHS/United States
- U19 AI135976/AI/NIAID NIH HHS/United States
- S10 OD018220/OD/NIH HHS/United States
- R01 CA142635/CA/NCI NIH HHS/United States
- P01 AI131374/AI/NIAID NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- U2C CA233238/CA/NCI NIH HHS/United States
- U2C CA233195/CA/NCI NIH HHS/United States
- R01 HL128173/HL/NHLBI NIH HHS/United States
- R33 CA183692/CA/NCI NIH HHS/United States
- T32 AR007422/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

