The S1P-S1PR Axis in Neurological Disorders-Insights into Current and Future Therapeutic Perspectives
- PMID: 32580348
- PMCID: PMC7349054
- DOI: 10.3390/cells9061515
The S1P-S1PR Axis in Neurological Disorders-Insights into Current and Future Therapeutic Perspectives
Abstract
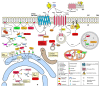
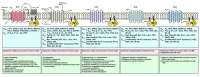
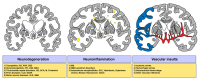
Sphingosine 1-phosphate (S1P), derived from membrane sphingolipids, is a pleiotropic bioactive lipid mediator capable of evoking complex immune phenomena. Studies have highlighted its importance regarding intracellular signaling cascades as well as membrane-bound S1P receptor (S1PR) engagement in various clinical conditions. In neurological disorders, the S1P-S1PR axis is acknowledged in neurodegenerative, neuroinflammatory, and cerebrovascular disorders. Modulators of S1P signaling have enabled an immense insight into fundamental pathological pathways, which were pivotal in identifying and improving the treatment of human diseases. However, its intricate molecular signaling pathways initiated upon receptor ligation are still poorly elucidated. In this review, the authors highlight the current evidence for S1P signaling in neurodegenerative and neuroinflammatory disorders as well as stroke and present an array of drugs targeting the S1P signaling pathway, which are being tested in clinical trials. Further insights on how the S1P-S1PR axis orchestrates disease initiation, progression, and recovery may hold a remarkable potential regarding therapeutic options in these neurological disorders.
Keywords: S1P1–5; fingolimod; multiple sclerosis; neurodegeneration; sphingosine 1-phoshate; sphingosine 1-phosphate antagonistst/inhibitors; sphingosine 1-phosphate metabolism; sphingosine 1-phosphate receptor; sphingosine 1-phosphate signaling; stroke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

