Progressive Skeletal Muscle Atrophy in Muscular Dystrophies: A Role for Toll-like Receptor-Signaling in Disease Pathogenesis
- PMID: 32580419
- PMCID: PMC7352931
- DOI: 10.3390/ijms21124440
Progressive Skeletal Muscle Atrophy in Muscular Dystrophies: A Role for Toll-like Receptor-Signaling in Disease Pathogenesis
Abstract
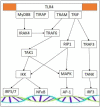
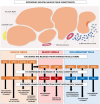
Muscle atrophy is an active process controlled by specific transcriptional programs, in which muscle mass is lost by increased protein degradation and/or decreased protein synthesis. This review explores the involvement of Toll-like receptors (TLRs) in the muscle atrophy as it is observed in muscular dystrophies, disorders characterized by successive bouts of muscle fiber degeneration and regeneration in an attempt to repair contraction-induced damage. TLRs are defense receptors that detect infection and recognize self-molecules released from damaged cells. In muscular dystrophies, these receptors become over-active, and are firmly involved in the sustained chronic inflammation exhibited by the muscle tissue, via their induction of pro-inflammatory cytokine expression. Taming the exaggerated activation of TLR2/4 and TLR7/8/9, and their downstream effectors in particular, comes forward as a therapeutic strategy with potential to slow down disease progression.
Keywords: Toll-like receptors; muscular dystrophies; myositis.
Conflict of interest statement
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Mouisel E., Vignaud A., Hourde C., Butler-Browne G., Ferry A. Muscle weakness and atrophy are associated with decreased regenerative capacity and changes in MTOR signaling in skeletal muscle of vednerable (18-24-month-old) dystrophic mdx mice. Muscle Nerve. 2010;41:809–818. doi: 10.1002/mus.21624. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

