The Cerebral Localization of Pain: Anatomical and Functional Considerations for Targeted Electrical Therapies
- PMID: 32580436
- PMCID: PMC7355617
- DOI: 10.3390/jcm9061945
The Cerebral Localization of Pain: Anatomical and Functional Considerations for Targeted Electrical Therapies
Abstract
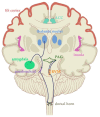
Millions of people in the United States are affected by chronic pain, and the financial cost of pain treatment is weighing on the healthcare system. In some cases, current pharmacological treatments may do more harm than good, as with the United States opioid crisis. Direct electrical stimulation of the brain is one potential non-pharmacological treatment with a long history of investigation. Yet brain stimulation has been far less successful than peripheral or spinal cord stimulation, perhaps because of our limited understanding of the neural circuits involved in pain perception. In this paper, we review the history of using electrical stimulation of the brain to treat pain, as well as contemporary studies identifying the structures involved in pain networks, such as the thalamus, insula, and anterior cingulate. We propose that the thermal grill illusion, an experimental pain model, can facilitate further investigation of these structures. Pairing this model with intracranial recording will provide insight toward disentangling the neural correlates from the described anatomic areas. Finally, the possibility of altering pain perception with brain stimulation in these regions could be highly informative for the development of novel brain stimulation therapies for chronic pain.
Keywords: closed loop; deep brain stimulation; electrophysiology; imaging; neurophysiology; pain; sensing; thermal grill.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Institute of Medicine . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; Washington, DC, USA: 2011. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

