Clinical and Analytical Performance of an Automated Serological Test That Identifies S1/S2-Neutralizing IgG in COVID-19 Patients Semiquantitatively
- PMID: 32580948
- PMCID: PMC7448652
- DOI: 10.1128/JCM.01224-20
Clinical and Analytical Performance of an Automated Serological Test That Identifies S1/S2-Neutralizing IgG in COVID-19 Patients Semiquantitatively
Abstract
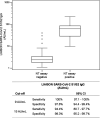
In the coronavirus (CoV) disease 2019 (COVID-19) pandemic, highly selective serological testing is essential to define exposure to severe acute respiratory syndrome CoV 2 (SARS-CoV-2). Many tests have been developed, yet with variable speeds to first results, and are of unknown quality, particularly when considering the prediction of neutralizing capacity. The LIAISON SARS-CoV-2 S1/S2 IgG assay was designed to measure antibodies against the SARS-CoV-2 native S1/S2 proteins in a standardized automated chemiluminescence assay. The clinical and analytical performances of the test were validated in an observational study using residual samples (>1,500) with a positive or negative COVID-19 diagnosis. The LIAISON SARS-CoV-2 S1/S2 IgG assay proved to be highly selective and specific and offered semiquantitative measures of serum or plasma levels of anti-S1/S2 IgG with neutralizing activity. The assay's diagnostic sensitivities were 91.3% and 95.7% at >5 or ≥15 days from diagnosis, respectively, and 100% when assessed against a neutralizing assay. The assay's specificity ranged between 97% and 98.5%. The average imprecision of the assay was a <5% coefficient of variation. Assay performance at 2 different cutoffs was evaluated to optimize predictive values. The automated LIAISON SARS-CoV-2 S1/S2 IgG assay brings efficient, sensitive, specific, and precise serological testing to the laboratory, with the capacity to test large amounts of samples per day; first results are available within 35 min, with a throughput of 170 tests/hour. The semiquantitative results provided by the test also associate with the presence of neutralizing antibodies and may provide a useful tool for the large-scale screening of convalescent-phase plasma for safe therapeutic use.
Keywords: CLIA; COVID-19; SARS-CoV-2; diagnostics; immunoassays; immunoserology; neutralization assay; neutralizing antibodies; spike.
Copyright © 2020 Bonelli et al.
Figures



References
-
- Reusken CB, Broberg EK, Haagmans B, Meijer A, Corman VM, Papa A, Charrel R, Drosten C, Koopmans M, Leitmeyer K, on behalf of EVD-LabNet and ERLI-Net. 2020. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020. Eurosurveillance 25:2000082. doi: 10.2807/1560-7917.ES.2020.25.6.2000082. - DOI - PMC - PubMed
-
- Johns Hopkins University, Coronavirus Resource Center. 2020. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE). Johns Hopkins University, Baltimore, MD: https://coronavirus.jhu.edu/map.html. Accessed 18 April 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

