CD200 promotes immunosuppression in the pancreatic tumor microenvironment
- PMID: 32581043
- PMCID: PMC7312341
- DOI: 10.1136/jitc-2019-000189
CD200 promotes immunosuppression in the pancreatic tumor microenvironment
Abstract
Background: A significant challenge to overcome in pancreatic ductal adenocarcinoma (PDAC) is the profound systemic immunosuppression that renders this disease non-responsive to immunotherapy. Our supporting data provide evidence that CD200, a regulator of myeloid cell activity, is expressed in the PDAC microenvironment. Additionally, myeloid-derived suppressor cells (MDSC) isolated from patients with PDAC express elevated levels of the CD200 receptor (CD200R). Thus, we hypothesize that CD200 expression in the PDAC microenvironment limits responses to immunotherapy by promoting expansion and activity of MDSC.
Methods: Immunofluorescent staining was used to determine expression of CD200 in murine and human PDAC tissue. Flow cytometry was utilized to test for CD200R expression by immune populations in patient blood samples. In vivo antibody blocking of CD200 was conducted in subcutaneous MT-5 tumor-bearing mice and in a genetically engineered PDAC model (KPC-Brca2 mice). Peripheral blood mononuclear cells (PBMC) from patients with PDAC were analyzed by single-cell RNA sequencing. MDSC expansion assays were completed using healthy donor PBMC stimulated with IL-6/GM-CSF in the presence of recombinant CD200 protein.
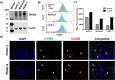
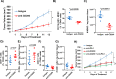
Results: We found expression of CD200 by human pancreatic cell lines (BxPC3, MiaPaca2, and PANC-1) as well as on primary epithelial pancreatic tumor cells and smooth muscle actin+ stromal cells. CD200R expression was found to be elevated on CD11b+CD33+HLA-DRlo/- MDSC immune populations from patients with PDAC (p=0.0106). Higher expression levels of CD200R were observed in CD15+ MDSC compared with CD14+ MDSC (p<0.001). In vivo studies demonstrated that CD200 antibody blockade limited tumor progression in MT-5 subcutaneous tumor-bearing and in KPC-Brca2 mice (p<0.05). The percentage of intratumoral MDSC was significantly reduced in anti-CD200 treated mice compared with controls. Additionally, in vivo blockade of CD200 can also significantly enhance the efficacy of PD-1 checkpoint antibodies compared with single antibody therapies (p<0.05). Single-cell RNA sequencing of PBMC from patients revealed that CD200R+ MDSC expressed genes involved in cytokine signaling and MDSC expansion. Further, in vitro cytokine-driven expansion and the suppressive activity of human MDSC was enhanced when cocultured with recombinant CD200 protein.
Conclusions: These results indicate that CD200 expression in the PDAC microenvironment may regulate MDSC expansion and that targeting CD200 may enhance activity of checkpoint immunotherapy.
Keywords: gastroenterology; immunology; myeloid-derived suppressor cells; tumor microenvironment; tumors.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
