Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study
- PMID: 32581057
- PMCID: PMC7319717
- DOI: 10.1136/jitc-2019-000331
Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study
Erratum in
-
Correction: Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study.J Immunother Cancer. 2020 Nov;8(2):e000331corr1. doi: 10.1136/jitc-2019-000331corr1. J Immunother Cancer. 2020. PMID: 33229507 Free PMC article. No abstract available.
Abstract
Background: Uveal melanoma (UM) is the most common intraocular malignancy in adults. In contrast to cutaneous melanoma (CM), there is no standard therapy, and the efficacy and safety of dual checkpoint blockade with nivolumab and ipilimumab is not well defined.
Methods: We conducted a retrospective analysis of patients with metastatic UM (mUM) who received treatment with ipilimumab plus nivolumab across 14 academic medical centers. Toxicity was graded using National Cancer Institute Common Terminology Criteria for Adverse Events V.5.0. Progression-free survival (PFS) and overall survival (OS) were calculated using Kaplan-Meier methodology.
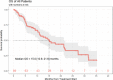
Results: 89 eligible patients were identified. 45% had received prior therapy, which included liver directed therapy (29%), immunotherapy (21%), targeted therapy (10%) and radiation (16%). Patients received a median 3 cycles of ipilimumab plus nivolumab. The median follow-up time was 9.2 months. Overall response rate was 11.6%. One patient achieved complete response (1%), 9 patients had partial response (10%), 21 patients had stable disease (24%) and 55 patients had progressive disease (62%). Median OS from treatment initiation was 15 months and median PFS was 2.7 months. Overall, 82 (92%) of patients discontinued treatment, 34 due to toxicity and 27 due to progressive disease. Common immune-related adverse events were colitis/diarrhea (32%), fatigue (23%), rash (21%) and transaminitis (21%).
Conclusions: Dual checkpoint inhibition yielded higher response rates than previous reports of single-agent immunotherapy in patients with mUM, but the efficacy is lower than in metastatic CM. The median OS of 15 months suggests that the rate of clinical benefit may be larger than the modest response rate.
Keywords: immunotherapy; melanoma; oncology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: YGN: Research funding: Merck, Pfizer, BMS. Advisory Board: Array. KKS: Institutional funding: Oncosec, Regeneron. ZE: Research support: Novartis. Advisory board: Array, Regeneron. SC: Advisory Board and (non branded) Speaker’s Bureau for BMS. RC: Consulting: Array, BMS, Castle Biosciences, Compugen, Immunocore, I-Mab, InxMed, Merck, Roche/Genentech, Pierre Fabre, PureTech Health, Sanofi Genzyme, Sorrento Therapeutics. Clinical/Scientific Advisory Boards: Aura Biosciences, Chimeron, Rgenix. Research Funding to Columbia University: Amgen, Array, Astellis, AstraZeneca, Bayer, Bellicum, BMS, Corvus, Eli Lilly, Immunocore, Incyte, Macrogenics, Merck, Mirati, Novartis, Pfizer, Plexxikon, Roche/Genentech. JMK: Grants and personal fees from Bristol‐Myers Squibb and Immunocore; personal fees from Novartis, Iovance, and Elsevier; grants from Checkmate and Merck; Consulting or advisory role for Bristol‐Myers Squibb, Novartis, Array BioPharma, Merck, Roche, Amgen, and Immunocore. RS: Consulting/Advisory Boards: Amgen, Array, BMS, Merck, Novartis, Genentech, Compugen, Replimmune. Research support: Merck, Amgen. DJ: Advisory boards for Array Biopharma, Bristol-Myers Squibb, Incyte, Merck, Novartis, and Genoptix. Research support from Bristol-Myers Squibb and Incyte. AS: Advisory Board: Bristol-Myers Squibb, Immunocore, Castle Biosciences Institutional Research Support: Bristol-Myers Squibb, Immunocore, XcoveryTravel: Parker Institute for Cancer Immunotherapy.
Figures



References
-
- Chang AE, Karnell LH, Menck HR. The National cancer data base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. the American College of surgeons Commission on cancer and the American cancer Society. Cancer 1998;83:1664–78. 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g - DOI - PubMed
