Residues within the Foot-and-Mouth Disease Virus 3Dpol Nuclear Localization Signal Affect Polymerase Fidelity
- PMID: 32581111
- PMCID: PMC7431809
- DOI: 10.1128/JVI.00833-20
Residues within the Foot-and-Mouth Disease Virus 3Dpol Nuclear Localization Signal Affect Polymerase Fidelity
Abstract
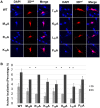
Many RNA viruses encode a proof-reading deficient, low-fidelity RNA-dependent polymerase (RdRp), which generates genetically diverse populations that can adapt to changing environments and thwart antiviral therapies. 3Dpol, the RdRp of the foot-and-mouth disease virus (FMDV), is responsible for replication of viral genomes. The 3Dpol N terminus encodes a nuclear localization signal (NLS) sequence,MRKTKLAPT, important for import of the protein to host nucleus. Previous studies showed that substitutions at residues 18 and 20 of the NLS are defective in proper incorporation of nucleotides and RNA binding. Here, we use a systematic alanine scanning mutagenesis approach to understand the role of individual residues of the NLS in nuclear localization and nucleotide incorporation activities of 3Dpol We identify two residues of 3Dpol NLS, T19 and L21, that are important for the maintenance of enzyme fidelity. The 3Dpol NLS alanine substitutions of T19 and L21 results in aberrant incorporation of nucleoside analogs, conferring a low fidelity phenotype of the enzyme. A molecular dynamics simulation of RNA- and mutagen (RTP)-bound 3Dpol revealed that the T19 residue participates in a hydrogen bond network, including D165 in motif F and R416 at the C terminus of the FMDV 3Dpol and RNA template-primer. Based on these findings and previous studies, we conclude that at least the first six residues of theMRKTKLAPT sequence motif play a vital role in the maintenance of faithful RNA synthesis activity (fidelity) of FMDV 3Dpol, suggesting that the role of the NLS motif in similar viral polymerases needs to be revisited.IMPORTANCE In this study, we employed genetic and molecular dynamics approaches to analyze the role of individual amino acids of the FMDV 3Dpol nuclear localization signal (NLS). The NLS residues were mutated to alanine using a type A full-genome cDNA clone, and the virus progeny was analyzed for defects in growth and in competition with the parental virus. We identified two mutants in 3Dpol, T19A and L21A, that exhibited high rate of mutation, were sensitive to nucleotide analogs, and displayed reduced replicative fitness compared to the parental virus. Using molecular dynamics simulation, we demonstrated that residues T19 and L21 played a role in the structural configuration of the interaction network at the 3Dpol palm subdomain. Cumulatively, our data suggest that the T19 and L21 3Dpol amino acids are important for maintaining the fidelity of the FMDV polymerase and ensuring faithful replication of the FMDV genome.
Keywords: 3Dpol; FMDV; RNA-dependent RNA polymerase fidelity; enzyme nuclear localization signal; foot-and-mouth disease virus; picornavirus.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

