The role of mPFC and MTL neurons in human choice under goal-conflict
- PMID: 32581214
- PMCID: PMC7314808
- DOI: 10.1038/s41467-020-16908-z
The role of mPFC and MTL neurons in human choice under goal-conflict
Erratum in
-
Author Correction: The role of mPFC and MTL neurons in human choice under goal-conflict.Nat Commun. 2020 Aug 10;11(1):4047. doi: 10.1038/s41467-020-17982-z. Nat Commun. 2020. PMID: 32778724 Free PMC article.
Abstract
Resolving approach-avoidance conflicts relies on encoding motivation outcomes and learning from past experiences. Accumulating evidence points to the role of the Medial Temporal Lobe (MTL) and Medial Prefrontal Cortex (mPFC) in these processes, but their differential contributions have not been convincingly deciphered in humans. We detect 310 neurons from mPFC and MTL from patients with epilepsy undergoing intracranial recordings and participating in a goal-conflict task where rewards and punishments could be controlled or not. mPFC neurons are more selective to punishments than rewards when controlled. However, only MTL firing following punishment is linked to a lower probability for subsequent approach behavior. mPFC response to punishment precedes a similar MTL response and affects subsequent behavior via an interaction with MTL firing. We thus propose a model where approach-avoidance conflict resolution in humans depends on outcome value tagging in mPFC neurons influencing encoding of such value in MTL to affect subsequent choice.
Conflict of interest statement
The authors declare no competing interests.
Figures

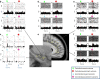
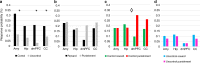
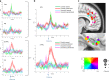

Comment in
-
The Neuroscientist Comments.Neuroscientist. 2021 Aug;27(4):318. doi: 10.1177/10738584211026888. Neuroscientist. 2021. PMID: 34229516 No abstract available.
Similar articles
-
Neural bases of behavior selection without an objective correct answer.Neurosci Lett. 2009 Jul 31;459(1):30-4. doi: 10.1016/j.neulet.2009.04.056. Epub 2009 May 4. Neurosci Lett. 2009. PMID: 19409960
-
Distinct medial temporal networks encode surprise during motivation by reward versus punishment.Neurobiol Learn Mem. 2016 Oct;134 Pt A(Pt A):55-64. doi: 10.1016/j.nlm.2016.01.018. Epub 2016 Feb 5. Neurobiol Learn Mem. 2016. PMID: 26854903 Free PMC article.
-
Neurons in rat orbitofrontal cortex and medial prefrontal cortex exhibit distinct responses in reward and strategy-update in a risk-based decision-making task.Metab Brain Dis. 2019 Apr;34(2):417-429. doi: 10.1007/s11011-018-0360-x. Epub 2018 Dec 8. Metab Brain Dis. 2019. PMID: 30535618
-
Rewards and punishments, goal-directed behavior and consciousness.Neurosci Biobehav Rev. 2004 Mar;28(1):27-39. doi: 10.1016/j.neubiorev.2003.10.003. Neurosci Biobehav Rev. 2004. PMID: 15036931 Review.
-
Integration of value and action in medial prefrontal neural systems.Int Rev Neurobiol. 2021;158:57-82. doi: 10.1016/bs.irn.2020.11.007. Epub 2020 Dec 17. Int Rev Neurobiol. 2021. PMID: 33785156 Free PMC article. Review.
Cited by
-
The geometry of domain-general performance monitoring in the human medial frontal cortex.Science. 2022 May 6;376(6593):eabm9922. doi: 10.1126/science.abm9922. Epub 2022 May 6. Science. 2022. PMID: 35511978 Free PMC article.
-
Hippocampal damage disrupts the latent decision-making processes underlying approach-avoidance conflict processing in humans.PLoS Biol. 2025 Feb 11;23(2):e3003033. doi: 10.1371/journal.pbio.3003033. eCollection 2025 Feb. PLoS Biol. 2025. PMID: 39932954 Free PMC article.
-
The development of aperiodic neural activity in the human brain.bioRxiv [Preprint]. 2025 Apr 16:2024.11.08.622714. doi: 10.1101/2024.11.08.622714. bioRxiv. 2025. Update in: Nat Hum Behav. 2025 Jul 21. doi: 10.1038/s41562-025-02270-x. PMID: 39574667 Free PMC article. Updated. Preprint.
-
The development of aperiodic neural activity in the human brain.Nat Hum Behav. 2025 Jul 21. doi: 10.1038/s41562-025-02270-x. Online ahead of print. Nat Hum Behav. 2025. PMID: 40670699
-
Neurons in human pre-supplementary motor area encode key computations for value-based choice.Nat Hum Behav. 2023 Jun;7(6):970-985. doi: 10.1038/s41562-023-01548-2. Epub 2023 Mar 23. Nat Hum Behav. 2023. PMID: 36959327 Free PMC article.
References
-
- Ranaldi R. Dopamine and reward seeking: the role of ventral tegmental area. Rev. Neurosci. 2014;25:621–630. - PubMed
-
- Feigley DA, Spear NE. Effect of age and punishment condition on long-term retention by the rat of active- and passive-avoidance learning. J. Comp. Physiol. Psychol. 1970;73:515–526. - PubMed
-
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 2000;23:473–500. - PubMed
-
- Matsumoto M, Matsumoto K, Abe H, Tanaka K. Medial prefrontal cell activity signaling prediction errors of action values. Nat. Neurosci. 2007;10:647. - PubMed
-
- Davidow JY, Foerde K, Galván A, Shohamy D. An upside to reward sensitivity: the hippocampus supports enhanced reinforcement learning in adolescence. Neuron. 2016;92:93–99. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

