Radiation-induced bystander and abscopal effects: important lessons from preclinical models
- PMID: 32581341
- PMCID: PMC7403362
- DOI: 10.1038/s41416-020-0942-3
Radiation-induced bystander and abscopal effects: important lessons from preclinical models
Abstract
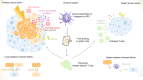
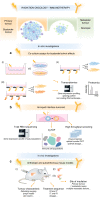
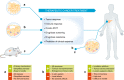
Radiotherapy is a pivotal component in the curative treatment of patients with localised cancer and isolated metastasis, as well as being used as a palliative strategy for patients with disseminated disease. The clinical efficacy of radiotherapy has traditionally been attributed to the local effects of ionising radiation, which induces cell death by directly and indirectly inducing DNA damage, but substantial work has uncovered an unexpected and dual relationship between tumour irradiation and the host immune system. In clinical practice, it is, therefore, tempting to tailor immunotherapies with radiotherapy in order to synergise innate and adaptive immunity against cancer cells, as well as to bypass immune tolerance and exhaustion, with the aim of facilitating tumour regression. However, our understanding of how radiation impacts on immune system activation is still in its early stages, and concerns and challenges regarding therapeutic applications still need to be overcome. With the increasing use of immunotherapy and its common combination with ionising radiation, this review briefly delineates current knowledge about the non-targeted effects of radiotherapy, and aims to provide insights, at the preclinical level, into the mechanisms that are involved with the potential to yield clinically relevant combinatorial approaches of radiotherapy and immunotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Nikjoo H, O’Neill P, Wilson WE, Goodhead DT. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat. Res. 2001;156:577–583. - PubMed
-
- Seymour CB, Mothersill C. Delayed expression of lethal mutations and genomic instability in the progeny of human epithelial cells that survived in a bystander-killing environment. Radiat. Oncol. Investig. 1997;5:106–110. - PubMed
-
- Blyth BJ, Sykes PJ. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat. Res. 2011;176:139–157. - PubMed
-
- Koturbash I, Loree J, Kutanzi K, Koganow C, Pogribny I, Kovalchuk O. In vivo bystander effect: cranial X-irradiation leads to elevated DNA damage, altered cellular proliferation and apoptosis, and increased p53 levels in shielded spleen. Int J. Radiat. Oncol. Biol. Phys. 2008;70:554–562. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

