Single-molecule imaging of transcription dynamics in somatic stem cells
- PMID: 32581360
- PMCID: PMC8577313
- DOI: 10.1038/s41586-020-2432-4
Single-molecule imaging of transcription dynamics in somatic stem cells
Abstract
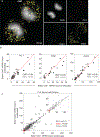
Molecular noise is a natural phenomenon that is inherent to all biological systems1,2. How stochastic processes give rise to the robust outcomes that support tissue homeostasis remains unclear. Here we use single-molecule RNA fluorescent in situ hybridization (smFISH) on mouse stem cells derived from haematopoietic tissue to measure the transcription dynamics of three key genes that encode transcription factors: PU.1 (also known as Spi1), Gata1 and Gata2. We find that infrequent, stochastic bursts of transcription result in the co-expression of these antagonistic transcription factors in the majority of haematopoietic stem and progenitor cells. Moreover, by pairing smFISH with time-lapse microscopy and the analysis of pedigrees, we find that although individual stem-cell clones produce descendants that are in transcriptionally related states-akin to a transcriptional priming phenomenon-the underlying transition dynamics between states are best captured by stochastic and reversible models. As such, a stochastic process can produce cellular behaviours that may be incorrectly inferred to have arisen from deterministic dynamics. We propose a model whereby the intrinsic stochasticity of gene expression facilitates, rather than impedes, the concomitant maintenance of transcriptional plasticity and stem cell robustness.
Conflict of interest statement
Figures







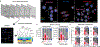

References
-
- Levsky JM & Singer RH Gene expression and the myth of the average cell. Trends Cell Biol. 13, 4–6 (2003). - PubMed
-
- Elowitz MB, Levine AJ, Siggia ED & Swain PS Stochastic gene expression in a single cell. Science 297, 1183–1186 (2002). - PubMed
-
- Bar-Even A et al. Noise in protein expression scales with natural protein abundance. Nature Genetics 38, 636–643 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

