Reversing a model of Parkinson's disease with in situ converted nigral neurons
- PMID: 32581380
- PMCID: PMC7521455
- DOI: 10.1038/s41586-020-2388-4
Reversing a model of Parkinson's disease with in situ converted nigral neurons
Erratum in
-
Author Correction: Reversing a model of Parkinson's disease with in situ converted nigral neurons.Nature. 2020 Aug;584(7820):E17. doi: 10.1038/s41586-020-2583-3. Nature. 2020. PMID: 32724206
Abstract
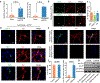
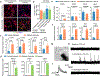
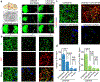
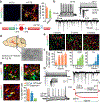
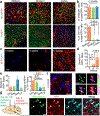
Parkinson's disease is characterized by loss of dopamine neurons in the substantia nigra1. Similar to other major neurodegenerative disorders, there are no disease-modifying treatments for Parkinson's disease. While most treatment strategies aim to prevent neuronal loss or protect vulnerable neuronal circuits, a potential alternative is to replace lost neurons to reconstruct disrupted circuits2. Here we report an efficient one-step conversion of isolated mouse and human astrocytes to functional neurons by depleting the RNA-binding protein PTB (also known as PTBP1). Applying this approach to the mouse brain, we demonstrate progressive conversion of astrocytes to new neurons that innervate into and repopulate endogenous neural circuits. Astrocytes from different brain regions are converted to different neuronal subtypes. Using a chemically induced model of Parkinson's disease in mouse, we show conversion of midbrain astrocytes to dopaminergic neurons, which provide axons to reconstruct the nigrostriatal circuit. Notably, re-innervation of striatum is accompanied by restoration of dopamine levels and rescue of motor deficits. A similar reversal of disease phenotype is also accomplished by converting astrocytes to neurons using antisense oligonucleotides to transiently suppress PTB. These findings identify a potentially powerful and clinically feasible approach to treating neurodegeneration by replacing lost neurons.
Conflict of interest statement
COMPETING INTERESTS
X-D. Fu is a founder of CurePharmacutics Inc.
Figures


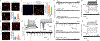


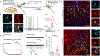





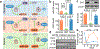



Comment in
-
Method to combat Parkinson's disease by astrocyte-to-neuron conversion.Nature. 2020 Jun;582(7813):489-490. doi: 10.1038/d41586-020-01817-4. Nature. 2020. PMID: 32581373 No abstract available.
-
Reversing a Model of Parkinson's Disease With In Situ Converted Nigral Neurons.Mov Disord. 2020 Nov;35(11):1955. doi: 10.1002/mds.28306. Epub 2020 Oct 5. Mov Disord. 2020. PMID: 33016569 No abstract available.
-
Ptbp1 deletion does not induce astrocyte-to-neuron conversion.Nature. 2023 Jun;618(7964):E1-E7. doi: 10.1038/s41586-023-06066-9. Epub 2023 Jun 7. Nature. 2023. PMID: 37286658 Free PMC article. No abstract available.
References
-
- Cohen DE & Melton D Turning straw into gold: directing cell fate for regenerative medicine. Nature Reviews Genetics 12, 243–252 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

