Analysis of Renal and Cardiac Outcomes in Male Participants in the Fabry Outcome Survey Starting Agalsidase Alfa Enzyme Replacement Therapy Before and After 18 Years of Age
- PMID: 32581513
- PMCID: PMC7276893
- DOI: 10.2147/DDDT.S249433
Analysis of Renal and Cardiac Outcomes in Male Participants in the Fabry Outcome Survey Starting Agalsidase Alfa Enzyme Replacement Therapy Before and After 18 Years of Age
Abstract
Purpose: To determine the impact of initiating enzyme replacement therapy (ERT) with agalsidase alfa early in the course of Fabry disease, we evaluated renal and cardiac outcomes for ≤10 years after ERT initiation in males from the Fabry Outcome Survey (FOS).
Patients and methods: Male patients from FOS were stratified into three cohorts by age at ERT initiation: ≤18 years (cohort 1), >18 and ≤30 years (cohort 2), and >30 years (cohort 3). Analysis included age at symptom onset, diagnosis, and ERT initiation; ERT duration; FOS-Mainz Severity Score Index (FOS-MSSI); estimated glomerular filtration rate (eGFR); proteinuria level; and left ventricular mass indexed to height (LVMI). Mixed-effect models estimated renal and cardiac outcomes during follow-up between and within cohorts.
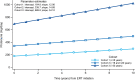
Findings: The analysis included 560 male patients: 151 (27.0%) in cohort 1, 155 (27.7%) in cohort 2, and 254 (45.4%) in cohort 3. Mean±SD duration of ERT for cohorts 1, 2, and 3 was 6.3±4.3, 8.6±4.9, and 7.9±4.9 years, respectively. Mean±SD baseline FOS-MSSI scores increased with age from 9.8±7.2 in cohort 1 to 24.7±11.4 in cohort 3. Cohort 3 showed the lowest baseline mean±SD value for eGFR (87.1±29.0 mL/min/1.73m2) and highest baseline mean±SD values for proteinuria (801.9±952.6 mg/day) and LVMI (56.7±16.0 g/m2.7) among the three cohorts. Evaluation of mean annual rates of change in eGFR, proteinuria, and LVMI revealed no significant differences in any parameter for cohort 1. For cohort 2, proteinuria and LVMI remained stable, whereas eGFR significantly deteriorated annually (-1.12 mL/min/1.73m2; P<0.001). Cohort 3 demonstrated significant annual deteriorations in eGFR (-2.60 mL/min/1.73m2; P<0.001), proteinuria (+34.10 mg/day; P<0.001), and LVMI (+0.59 g/m2.7; P=0.001).
Implications: Renal and/or cardiac disease progression appears attenuated in patients starting ERT in childhood or early adulthood versus patients starting ERT in later adulthood. These findings support early ERT initiation in Fabry disease. ClinicalTrials.gov identifier: NCT03289065.
Keywords: Fabry Outcome Survey; Fabry disease; agalsidase alfa; enzyme replacement therapy; estimated glomerular filtration rate; left ventricular hypertrophy.
© 2020 Parini et al.
Conflict of interest statement
Dr Parini reports personal fees and clinical trial participation from Shire, a Takeda company, and personal fees from BioMarin, Chiesi, Sanofi Genzyme, and Ultragenyx, outside the submitted work. Dr Pintos-Morrell reports personal fees from Alexion, Amicus, BioMarin, Kyowa-Kirin, Sanofi Genzyme, and Shire, a Takeda company, outside the submitted work. Dr Hennermann reports grants and personal fees from Shire, a Takeda company, and personal fees from Amicus, outside the submitted work. Dr Karabul reports grants and personal fees from Shire, a Takeda company, grants and personal fees from Sanofi Genzyme, and personal fees from BioMarin, outside the submitted work. Ms Kalampoki is an employee of and reports stock ownership from Shire, a Takeda company, during the conduct of the study. Dr Gurevich was an employee of Shire, a Takeda company, during the conduct of the study. Dr Ramaswami reports personal fees and clinical trial participation from Amicus, clinical trial participation from Protalix; grants, personal fees, and clinical trial participation from Sanofi Genzyme; grants, personal fees, and clinical trial participation from Shire, a Takeda company; and personal fees from Actelion, Idorsi, and Chiesi, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures


References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

