Sialylation and Galectin-3 in Microglia-Mediated Neuroinflammation and Neurodegeneration
- PMID: 32581723
- PMCID: PMC7296093
- DOI: 10.3389/fncel.2020.00162
Sialylation and Galectin-3 in Microglia-Mediated Neuroinflammation and Neurodegeneration
Abstract
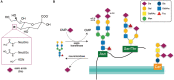
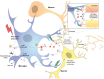
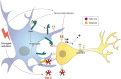
Microglia are brain macrophages that mediate neuroinflammation and contribute to and protect against neurodegeneration. The terminal sugar residue of all glycoproteins and glycolipids on the surface of mammalian cells is normally sialic acid, and addition of this negatively charged residue is known as "sialylation," whereas removal by sialidases is known as "desialylation." High sialylation of the neuronal cell surface inhibits microglial phagocytosis of such neurons, via: (i) activating sialic acid receptors (Siglecs) on microglia that inhibit phagocytosis and (ii) inhibiting binding of opsonins C1q, C3, and galectin-3. Microglial sialylation inhibits inflammatory activation of microglia via: (i) activating Siglec receptors CD22 and CD33 on microglia that inhibit phagocytosis and (ii) inhibiting Toll-like receptor 4 (TLR4), complement receptor 3 (CR3), and other microglial receptors. When activated, microglia release a sialidase activity that desialylates both microglia and neurons, activating the microglia and rendering the neurons susceptible to phagocytosis. Activated microglia also release galectin-3 (Gal-3), which: (i) further activates microglia via binding to TLR4 and TREM2, (ii) binds to desialylated neurons opsonizing them for phagocytosis via Mer tyrosine kinase, and (iii) promotes Aβ aggregation and toxicity in vivo. Gal-3 and desialylation may increase in a variety of brain pathologies. Thus, Gal-3 and sialidases are potential treatment targets to prevent neuroinflammation and neurodegeneration.
Keywords: aging; desialylation; galectin-3; microglia; neurodegeneration; phagocytosis; sialic acid.
Copyright © 2020 Puigdellívol, Allendorf and Brown.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

