Dl-3-n-Butylphthalide Promotes Remyelination and Suppresses Inflammation by Regulating AMPK/SIRT1 and STAT3/NF-κB Signaling in Chronic Cerebral Hypoperfusion
- PMID: 32581761
- PMCID: PMC7296049
- DOI: 10.3389/fnagi.2020.00137
Dl-3-n-Butylphthalide Promotes Remyelination and Suppresses Inflammation by Regulating AMPK/SIRT1 and STAT3/NF-κB Signaling in Chronic Cerebral Hypoperfusion
Abstract
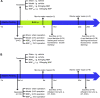
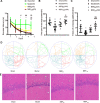
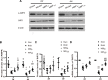
Demyelination in vascular dementia (VD) is partly attributable to inflammation induced by chronic cerebral hypoperfusion (CCH). Remyelination contributes to the recovery of cognitive impairment by inducing the proliferation and differentiation of oligodendrocyte progenitor cells. It was previously reported that Dl-3-n-butylphthalide (NBP) promotes cognitive improvement. However, whether NBP can stimulate remyelination and suppress inflammation after CCH remains unclear. To answer this question, the present study investigated the effects of NBP on remyelination in a rat model of CCH established by bilateral carotid artery occlusion. Functional recovery was evaluated with the Morris water maze (MWM) test, and myelin integrity, regeneration of mature oligodendrocytes, and inhibition of astrocyte proliferation were assessed by immunohistochemistry and histologic analysis. Additionally, activation of 5' AMP-activated protein kinase (AMPK)/Sirtuin (SIRT)1 and Signal transducer and activator of transcription (STAT)3/nuclear factor (NF)-κB signaling pathways was evaluated by western blotting. The results showed that NBP treatment improved memory and learning performance in CCH rats, which was accompanied by increased myelin integrity and oligodendrocyte regeneration, and reduced astrocyte proliferation and inflammation. Additionally, NBP induced the activation of AMPK/SIRT1 signaling while inhibiting the STAT3/NF-κB pathway. These results indicate that NBP alleviates cognitive impairment following CCH by promoting remyelination and suppressing inflammation via modulation of AMPK/SIRT1 and STAT3/NF-κB signaling.
Keywords: AMPK/SIRT1 pathway; Dl-3-n-butylphthalide (NBP); STAT3/NF-κB pathway; chronic cerebral hypoperfusion; inflammation; remyelination.
Copyright © 2020 Li, Meng, Guo, Niu, Zhao, Wang, Xie and Lv.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

