Prevention of Early Alzheimer's Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study
- PMID: 32581767
- PMCID: PMC7283924
- DOI: 10.3389/fnagi.2020.00155
Prevention of Early Alzheimer's Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study
Abstract
Objective: To investigate the efficacy and safety of three H. erinaceus mycelia (EAHE) capsules (350 mg/capsule; containing 5 mg/g erinacine A active ingredient) per day for the treatment of patients with mild Alzheimer's Disease (AD).
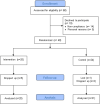
Methods: This study comprised a 3-week no-drug screening period, followed by a 49-week double-blind treatment period with 2-parallel groups in which eligible patients were randomized to either three 5 mg/g EAHE mycelia capsules per day or identical appearing placebo capsules. Cognitive assessments, ophthalmic examinations, biomarker collection, and neuroimaging were followed throughout the study period.
Results: After 49 weeks of EAHE intervention, a significant decrease in Cognitive Abilities Screening Instrument score was noted in the placebo group, a significant improvement in Mini-Mental State Examination score was observed in the EAHE group and a significant Instrumental Activities of Daily Living score difference were found between the two groups. In addition, EAHE group achieved a significantly better contrast sensitivity when compared to the placebo group. Moreover, only the placebo group observed significantly lowered biomarkers such as calcium, albumin, apolipoprotein E4, hemoglobin, and brain-derived neurotrophic factor and significantly elevated alpha1-antichymotrypsin and amyloid-beta peptide 1-40 over the study period. Using diffusion tensor imaging, the mean apparent diffusion coefficient (ADC) values from the arcuate fasciculus region in the dominant hemisphere significantly increased in the placebo group while no significant difference was found in the EAHE group in comparison to their baselines. Moreover, ADC values from the parahippocampal cingulum region in the dominant hemisphere significantly decreased in the EAHE group whereas no significant difference was found in the placebo group when compared to their baselines. Lastly, except for four subjects who dropped out of the study due to abdominal discomfort, nausea, and skin rash, no other adverse events were reported.
Conclusion: Three 350 mg/g EAHE capsules intervention for 49 weeks demonstrated higher CASI, MMSE, and IADL scores and achieved a better contrast sensitivity in patients with mild AD when compared to the placebo group, suggesting that EAHE is safe, well-tolerated, and may be important in achieving neurocognitive benefits.
Clinical trial registration: ClinicalTrials.gov, identifier NCT04065061.
Keywords: Alzheimer’s disease; erinacine A-enriched H. erinaceus mycelia; magnetic resonance imaging; pilot study; prevention.
Copyright © 2020 Li, Chang, Lin, Chen, Lu, Lee, Chen, Chen, Chen and Lin.
Figures
References
-
- Alzheimer’s Association, (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer Dement. 15 321–387.
-
- American Psychiatric Association, (2013). Diagnostic and Statistical Manual Of Mental Disorders: DSM-5, 5th Edn, Arlington, VA: American Psychiatric Publishing Inc.
-
- Andrieu S., Guyonnet S., Coley N., Cantet C., Bonnefoy M., Bordes S., et al. (2017). Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 16 377–389. 10.1016/S1474-4422(17)30040-6 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical