Immune Checkpoint Inhibitors and Immune-Related Adverse Drug Reactions: Data From Italian Pharmacovigilance Database
- PMID: 32581796
- PMCID: PMC7295943
- DOI: 10.3389/fphar.2020.00830
Immune Checkpoint Inhibitors and Immune-Related Adverse Drug Reactions: Data From Italian Pharmacovigilance Database
Abstract
Background: The introduction of immune checkpoint inhibitors (ICIs) in clinical practice has brought significant benefits for patients. Seven ICIs are available in Europe: nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, cemiplimab, and ipilimumab. Despite their proven clinical efficacy, these innovative drugs may cause serious immune-related adverse drugs reactions (irADRs). Given the significance of these ADRs for patients' health, we analyzed individual case safety reports (ICSRs) related to ICIs, focusing on those reporting irADRs, collected in the Italian spontaneous reporting database.
Methods: We analyzed ICI-induced irADRs collected in the Italian Pharmacovigilance database (Rete Nazionale di Farmacovigilanza [RNF]) from January 1, 2002, to February 28, 2019, focusing on those reported in the Campania Region. We retrieved from an open-access Italian pharmacovigilance system, the RAM system (for national safety data), and from the RNF (for Campania safety data) all ICSRs reporting ADRs related to ICIs authorized until the analysis date. Focusing on irADRs, we performed descriptive and disproportionality analyses through the reporting odds ratio (ROR) with 95% confidence interval.
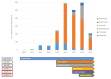
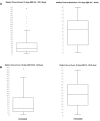
Results: National results. Among 2,088 ICI-related ICSRs, 801 reported irADRs. The majority of such ADRs occurred in male patients reporting gastrointestinal and skin toxicities. Nivolumab and pembrolizumab were drugs most commonly reported as suspect drugs. Compared to other ICIs, ROR was statistically significant for pembrolizumab and ipilimumab.Campania Region results. Out of 253 ICI-related ICSRs sent to Regional Pharmacovigilance Center of Campania Region, 121 reported at least one ICI-induced irADR. These were serious in 37.2% of cases and had an unfavorable outcome in 32.2% of cases. Overall, out of 8 ICSRs reported ADR with a fatal outcome, four reported irADRs. From disproportionality analyses on Campania Region ICSRs, statistically significant ROR emerged only for ipilimumab.
Conclusions: Our results showed that during the study period several serious irADRs were reported, some of which had fatal outcome. Given the clinical relevance of irADRs, further investigations in real-life context are necessary for a better characterization of ICIs safety profiles. Oncologists should be trained to early recognize and adequately manage irADRs. Patients should also be educated to immediately report any new symptom or worsening of pre-existed ones during the ICI treatment.
Keywords: immune checkpoint inhibitors; immune-related ADRs; pharmacovigilance; safety; spontaneous reporting system.
Copyright © 2020 Ruggiero, Fraenza, Scavone, di Mauro, Piscitelli, Mascolo, Ferrajolo, Rafaniello, Sportiello, Rossi and Capuano.
Figures


Similar articles
-
Pharmacovigilance study on the reporting frequency of atrial fibrillation with immune checkpoint inhibitors: insights from FDA Adverse Event Reporting System.Ther Adv Drug Saf. 2025 Apr 21;16:20420986241312497. doi: 10.1177/20420986241312497. eCollection 2025. Ther Adv Drug Saf. 2025. PMID: 40290514 Free PMC article.
-
Immune Checkpoint Inhibitors and Cardiotoxicity: An Analysis of Spontaneous Reports in Eudravigilance.Drug Saf. 2021 Sep;44(9):957-971. doi: 10.1007/s40264-021-01086-8. Epub 2021 Jun 18. Drug Saf. 2021. PMID: 34145536 Free PMC article.
-
Immune Checkpoint Inhibitors and Scleroderma: Data from the European Pharmacovigilance Database.Drugs Real World Outcomes. 2024 Mar;11(1):33-41. doi: 10.1007/s40801-023-00399-7. Epub 2023 Oct 31. Drugs Real World Outcomes. 2024. PMID: 37907712 Free PMC article.
-
Immune-related adverse events and immune checkpoint inhibitors: a focus on neurotoxicity and clinical management.Expert Rev Clin Pharmacol. 2023 May;16(5):423-434. doi: 10.1080/17512433.2023.2211262. Epub 2023 May 11. Expert Rev Clin Pharmacol. 2023. PMID: 37144360 Review.
-
PD-1/PD-L1 inhibitor-induced immune thrombocytopenia: A pharmacovigilance study and systematic review.Int Immunopharmacol. 2024 Mar 10;129:111606. doi: 10.1016/j.intimp.2024.111606. Epub 2024 Feb 14. Int Immunopharmacol. 2024. PMID: 38359661
Cited by
-
Comparative cardiotoxicity risk of pembrolizumab versus nivolumab in cancer patients undergoing immune checkpoint inhibitor therapy: A meta-analysis.Front Oncol. 2023 Mar 29;13:1080998. doi: 10.3389/fonc.2023.1080998. eCollection 2023. Front Oncol. 2023. PMID: 37064101 Free PMC article.
-
Machine-Learning Parsimonious Prediction Model for Diagnostic Screening of Severe Hematological Adverse Events in Cancer Patients Treated with PD-1/PD-L1 Inhibitors: Retrospective Observational Study by Using the Common Data Model.Diagnostics (Basel). 2025 Jan 20;15(2):226. doi: 10.3390/diagnostics15020226. Diagnostics (Basel). 2025. PMID: 39857110 Free PMC article.
-
Advances in Lung Cancer Treatment: Integrating Immunotherapy and Chinese Herbal Medicines to Enhance Immune Response.Chin J Integr Med. 2025 Sep;31(9):856-864. doi: 10.1007/s11655-025-4134-0. Epub 2025 May 23. Chin J Integr Med. 2025. PMID: 40407854 Review. No abstract available.
-
Nivolumab Safety in Renal Cell Carcinoma: A Case Report.J Pharm Technol. 2024 Apr;40(2):112-117. doi: 10.1177/87551225231218164. Epub 2023 Dec 15. J Pharm Technol. 2024. PMID: 38525093 Free PMC article.
-
The Reporting Frequency of Ketoacidosis Events with Dapagliflozin from the European Spontaneous Reporting System: The DAPA-KETO Study.Pharmaceuticals (Basel). 2022 Feb 25;15(3):286. doi: 10.3390/ph15030286. Pharmaceuticals (Basel). 2022. PMID: 35337085 Free PMC article.
References
-
- Brahmer J. R., Lacchetti C., Schneider B. J., Atkins M. B., Brassil K. J., Caterino J. M., et al. (2018). Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 36, 1714–1768. 10.1200/JCO.2017.77.6385 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

