Knowledge Mapping of Drug-Induced Liver Injury: A Scientometric Investigation (2010-2019)
- PMID: 32581801
- PMCID: PMC7291871
- DOI: 10.3389/fphar.2020.00842
Knowledge Mapping of Drug-Induced Liver Injury: A Scientometric Investigation (2010-2019)
Abstract
Background: Drug-induced liver injury (DILI) is a common adverse event, which compromises the safety of numerous drugs, poses a significant risk to patient health, and enhances healthcare expenditures. Many articles have been recently published on DILI related research, though no relevant scientometric study has been published yet. This scientometric study was aimed at comprehensively analyzing the knowledge base and emerging topics on DILI.
Methods: The articles and reviews related to DILI, published from 2010 to 2019 in the Web of Science Core Collection (WoSCC), were retrieved on March 15, 2020, using relevant keywords. Four different scientometric software (HistCite, VOSviewer, CiteSpace, and R-bibliometrix) was used to conduct this scientometric study.
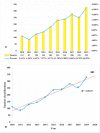
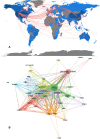
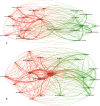
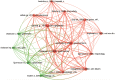
Results: A total of 1,995 publications were retrieved (including 1,550 articles and 445 reviews) from 592 academic journals with 56,273 co-cited references in 10 languages by 2,331 institutions from 79 countries/regions. The majority of publications (n = 727, 36.44%) were published in the United States, and the University of North Carolina contributed the most publications (n = 89, 4.46%). The most productive academic journal on DILI was the Toxicological Sciences [n = 79, 3.96%; impact factor (IF) 2018 = 3.564], and Hepatology was the first co-cited journal (n = 7,383, IF 2018 = 14.971). Fontana RJ and Teschke R may have significant influence on DILI research, with more publications (n = 46; n = 39) and co-citations (n = 382; n = 945). Definition, incidence rate or clinical characteristics, etiology or pathogenesis (such as the character of the innate immune system, the regulation of cell-death pathways, and susceptible HLA-B*5701 genotype), identification of main drugs and causality assessment (criteria and methods) were the knowledge base for DILI research. Exploring the microscopic mechanism (such as the organelle dysfunction and cytotoxicity induced by drugs, and exploration of role of neutrophils in DILI using mouse models) and developed newer approaches to prevent DILI (such as the prospective HLA-B*5701 screening and in vitro approaches for assessing the potential risk of candidate drugs for DILI) were the recent major topics for DILI research.
Conclusion: This scientometric study comprehensively reviewed the publications related to DILI during the past decade using quantitative and qualitative methods. This information would provide references for scholars, researching on DILI.
Keywords: CiteSpace; HLA-B*5701; VOSviewer; drug-induced liver injury; scientometric.
Copyright © 2020 Ke, Lu, Shen, Lu, Ma and Hua.
Figures




References
-
- Aria M., Cuccurullo C. (2017). bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 11 (4), 959–975. 10.1016/j.joi.2017.08.007 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

