Blood Flow Forces in Shaping the Vascular System: A Focus on Endothelial Cell Behavior
- PMID: 32581842
- PMCID: PMC7291788
- DOI: 10.3389/fphys.2020.00552
Blood Flow Forces in Shaping the Vascular System: A Focus on Endothelial Cell Behavior
Abstract
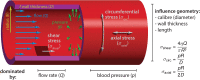
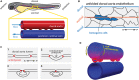
The endothelium is the cell monolayer that lines the interior of the blood vessels separating the vessel lumen where blood circulates, from the surrounding tissues. During embryonic development, endothelial cells (ECs) must ensure that a tight barrier function is maintained whilst dynamically adapting to the growing vascular tree that is being formed and remodeled. Blood circulation generates mechanical forces, such as shear stress and circumferential stretch that are directly acting on the endothelium. ECs actively respond to flow-derived mechanical cues by becoming polarized, migrating and changing neighbors, undergoing shape changes, proliferating or even leaving the tissue and changing identity. It is now accepted that coordinated changes at the single cell level drive fundamental processes governing vascular network morphogenesis such as angiogenic sprouting, network pruning, lumen formation, regulation of vessel caliber and stability or cell fate transitions. Here we summarize the cell biology and mechanics of ECs in response to flow-derived forces, discuss the latest advances made at the single cell level with particular emphasis on in vivo studies and highlight potential implications for vascular pathologies.
Keywords: Danio rerio (zebrafish); angiogenesis; cardiovascular; cilia; live imaging; low Reynolds number; mechanotransduction; stretch.
Copyright © 2020 Campinho, Vilfan and Vermot.
Figures

References
-
- Angulo-Urarte A., Casado P., Castillo S. D., Kobialka P., Kotini M. P., Figueiredo A. M., et al. (2018). Endothelial cell rearrangements during vascular patterning require PI3-kinase-mediated inhibition of actomyosin contractility. Nat. Commun. 9:4826. 10.1038/s41467-018-07172-3 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

