Involvement of Lipids in Alzheimer's Disease Pathology and Potential Therapies
- PMID: 32581851
- PMCID: PMC7296164
- DOI: 10.3389/fphys.2020.00598
Involvement of Lipids in Alzheimer's Disease Pathology and Potential Therapies
Abstract
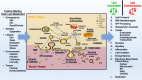
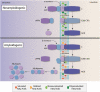
Lipids constitute the bulk of the dry mass of the brain and have been associated with healthy function as well as the most common pathological conditions of the brain. Demographic factors, genetics, and lifestyles are the major factors that influence lipid metabolism and are also the key components of lipid disruption in Alzheimer's disease (AD). Additionally, the most common genetic risk factor of AD, APOE ϵ4 genotype, is involved in lipid transport and metabolism. We propose that lipids are at the center of Alzheimer's disease pathology based on their involvement in the blood-brain barrier function, amyloid precursor protein (APP) processing, myelination, membrane remodeling, receptor signaling, inflammation, oxidation, and energy balance. Under healthy conditions, lipid homeostasis bestows a balanced cellular environment that enables the proper functioning of brain cells. However, under pathological conditions, dyshomeostasis of brain lipid composition can result in disturbed BBB, abnormal processing of APP, dysfunction in endocytosis/exocytosis/autophagocytosis, altered myelination, disturbed signaling, unbalanced energy metabolism, and enhanced inflammation. These lipid disturbances may contribute to abnormalities in brain function that are the hallmark of AD. The wide variance of lipid disturbances associated with brain function suggest that AD pathology may present as a complex interaction between several metabolic pathways that are augmented by risk factors such as age, genetics, and lifestyles. Herewith, we examine factors that influence brain lipid composition, review the association of lipids with all known facets of AD pathology, and offer pointers for potential therapies that target lipid pathways.
Keywords: amyloid precursor protein; apolipoproteins; blood-brain barrier; energy metabolism; inflammation; late-onset Alzheimer’s disease; mitochondria; myelination.
Copyright © 2020 Chew, Solomon and Fonteh.
Figures
Similar articles
-
Mass Spectrometry-Based Analysis of Lipid Involvement in Alzheimer's Disease Pathology-A Review.Metabolites. 2022 Jun 2;12(6):510. doi: 10.3390/metabo12060510. Metabolites. 2022. PMID: 35736443 Free PMC article. Review.
-
Role of amyloid beta in lipid homeostasis.Biochim Biophys Acta. 2010 Aug;1801(8):966-74. doi: 10.1016/j.bbalip.2010.05.002. Epub 2010 May 7. Biochim Biophys Acta. 2010. PMID: 20452461 Review.
-
Apolipoprotein E and Alzheimer's disease. A role in amyloid catabolism.Ann N Y Acad Sci. 2000;924:81-90. doi: 10.1111/j.1749-6632.2000.tb05564.x. Ann N Y Acad Sci. 2000. PMID: 11193807 Review.
-
ABCA7 Deficiency Accelerates Amyloid-β Generation and Alzheimer's Neuronal Pathology.J Neurosci. 2016 Mar 30;36(13):3848-59. doi: 10.1523/JNEUROSCI.3757-15.2016. J Neurosci. 2016. PMID: 27030769 Free PMC article.
-
Novel App knock-in mouse model shows key features of amyloid pathology and reveals profound metabolic dysregulation of microglia.Mol Neurodegener. 2022 Jun 11;17(1):41. doi: 10.1186/s13024-022-00547-7. Mol Neurodegener. 2022. PMID: 35690868 Free PMC article.
Cited by
-
PathFinder: a novel graph transformer model to infer multi-cell intra- and inter-cellular signaling pathways and communications.bioRxiv [Preprint]. 2024 Jan 15:2024.01.13.575534. doi: 10.1101/2024.01.13.575534. bioRxiv. 2024. Update in: Front Cell Neurosci. 2024 May 23;18:1369242. doi: 10.3389/fncel.2024.1369242. PMID: 38293243 Free PMC article. Updated. Preprint.
-
Statins and cognitive decline in patients with Alzheimer's and mixed dementia: a longitudinal registry-based cohort study.Alzheimers Res Ther. 2023 Dec 20;15(1):220. doi: 10.1186/s13195-023-01360-0. Alzheimers Res Ther. 2023. PMID: 38115091 Free PMC article.
-
'Fly-ing' from rare to common neurodegenerative disease mechanisms.Trends Genet. 2022 Sep;38(9):972-984. doi: 10.1016/j.tig.2022.03.018. Epub 2022 Apr 25. Trends Genet. 2022. PMID: 35484057 Free PMC article. Review.
-
Current and Emerging Therapies for Chronic Subjective Tinnitus.J Clin Med. 2023 Oct 16;12(20):6555. doi: 10.3390/jcm12206555. J Clin Med. 2023. PMID: 37892692 Free PMC article. Review.
-
Vitamin B12 Attenuates Changes in Phospholipid Levels Related to Oxidative Stress in SH-SY5Y Cells.Cells. 2022 Aug 18;11(16):2574. doi: 10.3390/cells11162574. Cells. 2022. PMID: 36010649 Free PMC article.
References
-
- Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2010). Structure and function of the blood-brain barrier. Neurobiol. Dis. 37 13–25. - PubMed
-
- Agrawal M., Ajazuddin, Tripathi D. K., Saraf S., Saraf S., Antimisiaris S. G., et al. (2017). Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 260 61–77. 10.1016/j.jconrel.2017.05.019 - DOI - PubMed
-
- Alexander G. E. (2017). An Emerging role for imaging white matter in the preclinical risk for Alzheimer disease: linking beta-amyloid to myelin. JAMA Neurol. 74 17–19. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

