Effect of Jian-Pi-Zhi-Dong Decoction on the Amino Acid Neurotransmitters in a Rat Model of Tourette Syndrome and Comorbid Anxiety Disorder
- PMID: 32581885
- PMCID: PMC7292006
- DOI: 10.3389/fpsyt.2020.00515
Effect of Jian-Pi-Zhi-Dong Decoction on the Amino Acid Neurotransmitters in a Rat Model of Tourette Syndrome and Comorbid Anxiety Disorder
Abstract
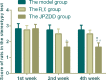
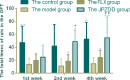
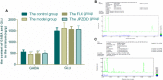
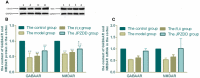
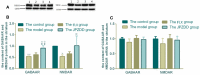
Amino acid neurotransmitters have been shown to correlate with Tourette syndrome (TS) and its comorbidities. In this study, we investigated the effects of Jian-Pi-Zhi-Dong Decoction (JPZDD), a formula containing 10 different Chinese medical herbs, on amino acid neurotransmitters in rats. We established a rat model of Tourette syndrome and comorbid anxiety with an iminodipropionitrile injection plus uncertain empty water bottle stimulation for 3 weeks. Then the rats were randomly divided into four groups: control group and model group were gavaged with saline, while the remaining two treatment groups were gavaged with fluoxetine hydrochloride or JPZDD for four consecutive weeks. We recorded the behaviors of the rats with TS and comorbid anxiety by stereotypy recording, open field test, and elevated plus maze. We observed mitochondrial changes with transmission electron microscopy. We measured the content of glutamate (GLU) and γ-aminobutyric acid (GABA) both in the serum and striatum and the expression of their receptors by Western blot and real-time polymerase chain reaction. The study revealed that JPZDD was effective in alleviating the behavioral symptoms of both tic and anxiety in the rat model groups. These results might be associated with the increase in GABA levels and decrease in GLU levels in the serum, as well as an increase in striatal GABA level by the activation of GABA receptors Type A (GABAAR). JPZDD treatment also reversed the mitochondrial dysfunction both in the striatum and cortex in affected animals.
Keywords: GABA receptors Type A; Jian-Pi-Zhi-Dong Decoction; N-methyl-D-aspartate receptor; Tourette syndrome; Traditional Chinese Medicine; comorbidity; glutamate; γ-aminobutyric acid.
Copyright © 2020 Zhang, Yu, Liu, Wang, Bai, Cui and Wang.
Figures
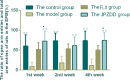


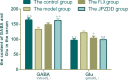
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

