The Clinical Value of 18 F-FDG-PET in Autoimmune Encephalitis Associated With LGI1 Antibody
- PMID: 32581996
- PMCID: PMC7290050
- DOI: 10.3389/fneur.2020.00418
The Clinical Value of 18 F-FDG-PET in Autoimmune Encephalitis Associated With LGI1 Antibody
Abstract
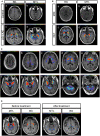
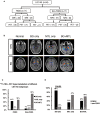
Purpose: The metabolic patterns of 18F-fluoro-2-deoxy-d-glucose positron emission tomography (18F-FDG-PET) in autoimmune encephalitis associated with leucine-rich glioma-inactivated 1 antibody (LGI1 AE) are still unclear. We performed a cohort study to investigate the clinical metabolic characteristics and diagnostic value based on 18F-FDG-PET in patients with LGI1 AE. Materials and Methods: A total of 34 patients including 18 patients (53%) in the acute phase and 16 patients (47%) in the chronic phase who were diagnosed with LGI1 AE were retrospectively analyzed from October 2014 to June 2018 at the Department of Neurology in Beijing Tiantan Hospital, the Capital Medical University. The clinical data were collected by searching through electronic medical records. Results: The initial 18F-FDG-PET scan indicated a significant abnormal metabolic pattern in 31 LGI1 AE patients (91%), whereas only 20 patients (59%) showed an abnormal MRI signal (P < 0.05). The 18F-FDG-PET metabolic pattern was reversible after treatment; most of the patients showed an almost normal uptake of 18F-FDG-PET after discharge. Regarding the spatial distribution, the abnormal metabolic pattern in LGI1 AE subjects exhibiting hypermetabolism was specifically located in the basal ganglia (BG) and medial temporal lobe (MTL). BG hypermetabolism was observed in 28 subjects (82%), and 68% of patients showed MTL hypermetabolism. A total of 17 patients (50%) exhibited faciobrachial dystonic seizures (FBDS), and the remaining subjects showed non-FBDS symptoms (50 and 50%). BG-only hypermetabolism was detected in seven subjects in the FBDS subgroup (7/16) but in only one subject in the non-FBDS subgroup (1/15) (44 vs. 7%, P < 0.05). Conclusion: 18F-FDG-PET imaging was more sensitive than MRI in the diagnosis of LGI1 AE. Isolated BG hypermetabolism was more frequently observed in subjects with FBDS, suggesting the potential involvement of the BG.
Keywords: 18F-FDG-PET; FBDS; LGI1; basal ganglia; medial temporal lobe.
Copyright © 2020 Liu, Shan, Zhao, Ren, Ren, Chen, Shi, Lv, Li, Liu, Ai and Wang.
Figures



References
LinkOut - more resources
Full Text Sources

