P2Y2 Receptor Promotes High-Fat Diet-Induced Obesity
- PMID: 32582029
- PMCID: PMC7283874
- DOI: 10.3389/fendo.2020.00341
P2Y2 Receptor Promotes High-Fat Diet-Induced Obesity
Abstract
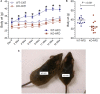
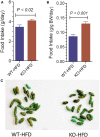
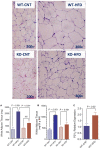
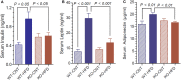
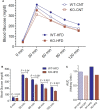
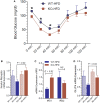
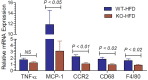
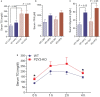
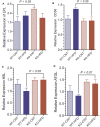
P2Y2, a G protein-coupled receptor (R), is expressed in all organs involved in the development of obesity and insulin resistance. To explore the role of it in diet-induced obesity, we fed male P2Y2-R whole body knockout (KO) and wild type (WT) mice (B6D2 genetic background) with regular diet (CNT; 10% calories as fat) or high-fat diet (HFD; 60% calories as fat) with free access to food and water for 16 weeks, and euthanized them. Adjusted for body weights (BW), KO mice consumed modestly, but significantly more HFD vs. WT mice, and excreted well-formed feces with no taint of fat or oil. Starting from the 2nd week, HFD-WT mice displayed significantly higher BW with terminal mean difference of 22% vs. HFD-KO mice. Terminal weights of white adipose tissue (WAT) were significantly lower in the HFD-KO vs. HFD-WT mice. The expression of P2Y2-R mRNA in WAT was increased by 2-fold in HFD-fed WT mice. Serum insulin, leptin and adiponectin levels were significantly elevated in the HFD-WT mice, but not in the HFD-KO mice. When induced in vitro, preadipocytes derived from KO mice fed regular diet did not differentiate and mature as robustly as those from the WT mice, as assessed by cellular expansion and accumulation of lipid droplets. Blockade of P2Y2-R by AR-C118925 in preadipocytes derived from WT mice prevented differentiation and maturation. Under basal conditions, KO mice had significantly higher serum triglycerides and showed slightly impaired lipid tolerance as compared to the WT mice. HFD-fed KO mice had significantly better glucose tolerance (GTT) as compared to HFD-fed WT mice. Whole body insulin sensitivity and mRNA expression of insulin receptor, IRS-1 and GLUT4 in WAT was significantly higher in HFD-fed KO mice vs. HFD-fed WT mice. On the contrary, the expression of pro-inflammatory molecules MCP-1, CCR2, CD68, and F4/80 were significantly higher in the WAT of HFD-fed WT vs. HFD-fed KO mice. These data suggest that P2Y2-R plays a significant role in the development of diet-induced obesity by promoting adipogenesis and inflammation, and altering the production of adipokines and lipids and their metabolism in adipose tissue, and thereby facilitates HFD-induced insulin resistance.
Keywords: AR-C 118925; adipose tissue; glucose homeostasis; inflammation; insulin resistance; lipid tolerance; obesity; purinergic signaling.
Copyright © 2020 Zhang, Ecelbarger, Lesniewski, Müller and Kishore.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

