Cadmium and Copper Cross-Tolerance. Cu+ Alleviates Cd2 + Toxicity, and Both Cations Target Heme and Chlorophyll Biosynthesis Pathway in Rubrivivax gelatinosus
- PMID: 32582041
- PMCID: PMC7283390
- DOI: 10.3389/fmicb.2020.00893
Cadmium and Copper Cross-Tolerance. Cu+ Alleviates Cd2 + Toxicity, and Both Cations Target Heme and Chlorophyll Biosynthesis Pathway in Rubrivivax gelatinosus
Abstract
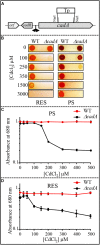
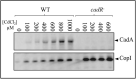
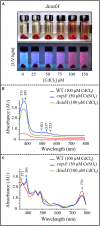
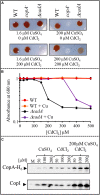
Cadmium, although not redox active is highly toxic. Yet, the underlying mechanisms driving toxicity are still to be characterized. In this study, we took advantage of the purple bacterium Rubrivivax gelatinosus strain with defective Cd2 +-efflux system to identify targets of this metal. Exposure of the ΔcadA strain to Cd2 + causes a decrease in the photosystem amount and in the activity of respiratory complexes. As in case of Cu+ toxicity, the data indicated that Cd2 + targets the porphyrin biosynthesis pathway at the level of HemN, a S-adenosylmethionine and CxxxCxxC coordinated [4Fe-4S] containing enzyme. Cd2 + exposure therefore results in a deficiency in heme and chlorophyll dependent proteins and metabolic pathways. Given the importance of porphyrin biosynthesis, HemN represents a key metal target to account for toxicity. In the environment, microorganisms are exposed to mixture of metals. Nevertheless, the biological effects of such mixtures, and the toxicity mechanisms remain poorly addressed. To highlight a potential cross-talk between Cd2 + and Cu+ -efflux systems, we show (i) that Cd2 + induces the expression of the Cd2 +-efflux pump CadA and the Cu+ detoxification system CopA and CopI; and (ii) that Cu+ ions improve tolerance towards Cd2 +, demonstrating thus that metal mixtures could also represent a selective advantage in the environment.
Keywords: CadA/ZntA; [4Fe-4S]; cadmium/copper; cross-talk; metal homeostasis; metal toxicity; porphyrin biosynthesis.
Copyright © 2020 Steunou, Durand, Bourbon, Babot, Tambosi, Liotenberg and Ouchane.
Figures





References
-
- Azzouzi A., Steunou A. S., Durand A., Khalfaoui-Hassani B., Bourbon M. L., Astier C., et al. (2013). Coproporphyrin III excretion identifies the anaerobic coproporphyrinogen III oxidase HemN as a copper target in the Cu+-ATPase mutant copA– of Rubrivivax gelatinosus. Mol. Microbiol. 88 339–351. 10.1111/mmi.12188 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

