The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages
- PMID: 32582063
- PMCID: PMC7296120
- DOI: 10.3389/fmicb.2020.01065
The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages
Abstract
Gut microbiota, an integral part of the human body, comprise bacteria, fungi, archaea, and protozoa. There is consensus that the disruption of the gut microbiota (termed "gut dysbiosis") is influenced by host genetics, diet, antibiotics, and inflammation, and it is closely linked to the pathogenesis of inflammatory diseases, such as obesity and inflammatory bowel disease (IBD). Macrophages are the key players in the maintenance of tissue homeostasis by eliminating invading pathogens and exhibit extreme plasticity of their phenotypes, such as M1 or M2, which have been demonstrated to exert pro- and anti-inflammatory functions. Microbiota-derived metabolites, short-chain fatty acids (SCFAs) and Gram-negative bacterial lipopolysaccharides (LPS), exert anti-inflammatory or pro-inflammatory effects by acting on macrophages. Understanding the role of macrophages in gut microbiota-inflammation interactions might provide us a novel method for preventing and treating inflammatory diseases. In this review, we summarize the recent research on the relationship between gut microbiota and inflammation and discuss the important role of macrophages in this context.
Keywords: gut microbiota; inflammatory bowel disease; inflammatory diseases; macrophage; obesity.
Copyright © 2020 Wang, Chen and Wang.
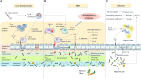
Figures
References
-
- Bain C. C., Scott C. L., Uronen-Hansson H., Gudjonsson S., Jansson O., Grip O., et al. (2013). Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6 498–510. 10.1038/mi.2012.89 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

